Abstract
This study tested the hypothesis that lipopolysaccharide (LPS) lowers arterial pressure through two different mechanisms depending on the dose. Previously, we found that a low hypotensive dose of LPS (1 mg/kg) lowers arterial pressure by activating vagus nerve afferents. Here we report that hypotension evoked by high dose LPS (15 mg/kg) can be prevented by injecting lidocaine into the OVLT but not by vagotomy or inactivation of the NTS. The hypotension produced by both LPS doses was correlated with elevated extracellular norepinephrine concentrations in the POA and prevented by blocking alpha-adrenergic receptors. Thus, initiation of endotoxic hypotension is dose-related, mechanistically.
Keywords: Endotoxin, lipopolysaccharide, septic shock, OVLT, preoptic area
1. INTRODUCTION
Septic shock has been characterized as a systemic inflammatory response to bacterial infection accompanied by persistent hypotension, tissue hypoperfusion and inadequate cellular oxygen utilization (Vincent, 2008; Angus and van der Poll, 2013). Although substantial progress has been made toward understanding the molecular pathophysiology of septic shock (Van Amersfoort et al., 2003; Angus and van der Poll, 2013; Bosmann and Ward, 2013; King et al., 2014) it continues to be a major cause of death and mortality rates remain unacceptably high (Martin et al., 2003; Jawad et al., 2012). Hence, there is a pressing need for new therapeutic strategies for treating, or preventing, septic shock.
Although hypotension, per se, does not fully account for the pathogenesis of septic shock, certainly restoration of arterial pressure and microvascular blood flow with intravenous fluids and vasopressors is standard initial therapy (Angus and van der Poll, 2013; Vincent and De Backer, 2013). Accordingly, treatments that prevent the initial fall in arterial pressure evoked by severe sepsis may, in theory, avoid some of its detrimental effects and reduce or eliminate requirements for resuscitation fluids and vasopressors. This consideration raises the question: What initiates the fall in arterial pressure during sepsis?
To address this question experimentally, we treated rats with lipopolysaccharide (LPS), a cell wall constituent of Gram-negative bacteria commonly used in experimental models of septic shock. It is often assumed that LPS lowers arterial pressure by stimulating the release of tumor necrosis factor-α (TNF-α) and other cytokines which act on the vasculature to produce vasodilation (Ulloa and Tracey, 2005). However, in earlier studies, we tested an alternative hypothesis; that endotoxic hypotension is initiated through a central mechanism (Yilmaz et al., 2008b). This hypothesis is based on evidence that LPS causes fever through a central mechanism in which the preoptic area of the hypothalamus (POA) plays a pivotal role (Blatteis et al., 2005). We found, as in the case of fever, that the fall in arterial pressure evoked by a relatively low hypotensive dose of LPS, 1 mg/kg i.v., could be prevented by microinjecting the local anesthetic lidocaine or the alpha-adrenergic receptor antagonist phentolamine into the POA of conscious or anesthetized rats (Yilmaz et al., 2008a; 2008b). Lipopolysaccharide administration also elevated extracellular norepinephrine (NE) concentrations in the POA (Villanueva et al., 2009), which suggests that LPS initially lowers arterial pressure by stimulating NE release. Together, these results indicate that endotoxic hypotension is initiated by activation of adrenergic neurons in the POA.
These findings raise a second fundamental question, how does a peripheral signal from LPS stimulate NE release within the POA? This issue has been studied extensively with regard to LPS-induced fever. LPS administration raises body temperature through two different mechanisms depending on the dose; low LPS doses (1 µg/kg i.v.) cause fever by activating vagus nerve afferents whereas higher doses (30 µg/kg i.v.) activate receptors in the organum vasculosum of the lamina terminalis (OVLT), the area postrema and, possibly, other circumventricular organs (Sehic and Blatteis, 1996; Azab and Kaplanski, 2001; Romanovsky et al., 2005).
The dose of LPS necessary to lower arterial pressure is considerably higher than the pyrogenic dose (Steiner et al., 2009) and it is unknown whether a similar dichotomous mechanism explains LPS-induced hypotension. In earlier studies we tested whether LPS lowers arterial pressure by activating vagus nerve afferents and found that either subdiaphragmatic vagotomy or lidocaine injection into the nucleus tractus solitarius (NTS), where vagal afferents synapse, prevented initiation of LPS hypotension completely (Yilmaz et al., 2008a). Lidocaine injection into either the OVLT or area postrema was ineffective, however. The fall in arterial pressure evoked by a relatively low hypotensive dose of LPS is thus initiated through vagus nerve afferents, not by activation of receptors in the OVLT or area postrema.
In this study we tested whether the hypotensive effect of a relatively high hypotensive dose of LPS, 15 mg/kg i.v., is initiated through the same, or a different, neural pathway. Here we report evidence that the cardiovascular effects of high dose LPS are mediated by the OVLT, but not the vagus nerve.
2. MATERIALS AND METHODS
Male Sprague-Dawley rats (250–300 g; Charles River Laboratories, Wilmington, MA) were anesthetized with 4% isoflurane and maintained with 1.5% isoflurane in 100% O2. The left femoral artery and left jugular vein were cannulated with PE-50 tubing filled with heparinized saline (100 U/ml) to record arterial pressure and administer drugs. At the beginning of each experiment the arterial cannula was connected to a volumetric pressure transducer attached to a DA100C transducer amplifier (BIOPAC Systems, CA, U.S.A.). Arterial blood pressure and heart rate were recorded using a Biopac MP150 system and AcqKnowledge software (BIOPAC Systems, CA, U.S.A.). The animal protocols were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
2.1. Microdialysis
A sterile, 17 mm long, 17-gauge, thin-walled stainless steel guide cannula with a tightly fitting indwelling stylette was implanted stereotaxically into the POA 0.3 mm caudal and 0.8 mm lateral to bregma and 8.5 mm below the dural surface (Paxinos and Watson, 2005) and fixed to the skull with four stainless steel screws and dental acrylic cement. Concentric microdialysis probes were constructed as previously described (Blatteis et al, 2005). Approximately two hours before each experiment the stylette was removed from the guide cannula and replaced with a sterile microdialysis probe with the tip of the dialysis membrane protruding 1 mm below the end of the guide cannula. The probe was fixed to the skull with tissue adhesive and perfused with sterile artificial cerebrospinal fluid (NaCl, 148 mM; KCl, 2.7 mM; CaCl2, 1.2 mM; MgCl2, 0.8 mM, pH 7.4), prepared fresh daily and pre-warmed to 38 °C, using sterile 1-ml tuberculin syringes clamped to a syringe pump (model #A-99; Razel Scientific Instruments, Stamford, CT, U.S.A.).
The microdialysis probes were perfused for a 90 min stabilization period at an initial flow rate of 4 µl/min for 10 min, then at 3 µl/min for 10 min, then at 2 µl/min for the remainder of the experiment. The microdialysis effluents were collected at 10 min intervals into ice cold polypropylene tubes containing 1 µl 5% perchloric acid and stored at −20 °C until analysis. At the end of each experiment the rat was euthanized with an overdose of isoflurane, the brain was rapidly removed, frozen on dry ice and sections (50 µm) were cut with a microtome cryostat. The sections were mounted on slides, stained with eosin, air dried, cover slipped and the location of the tip of the microdialysis probe was confirmed by visual inspection. Only data from confirmed probe placements were included.
2.2. Norepinephrine analysis
The NE concentration of microdialysis samples was evaluated using HPLC with electrochemical detection (Villanueva et al., 2009). Samples (5 µl) were injected using a CMA 200 refrigerated automatic sampler (CMA Microdialysis, North Chelmsford, MA, U.S.A.) onto a 150 × 3 mm ODS C18 column (ESA, Inc., Bedford, MA, U.S.A.) perfused by BAS 200A HPLC pumps (BAS, Inc., West Lafayette, IN, U.S.A.) at 0.25 ml/min with a mobile phase containing 80 mM sodium dihydrogen phosphate monohydrate, 2.0 mM 1-octanesulfonic acid sodium salt, 100 µl/liter triethylamine, 5 nM EDTA and 10% acetonitrile, pH 3.0. Norepinephrine was analyzed by using an ESA Coulochem II 5200A electrochemical detector with an ESA 5041 high-sensitivity microbore analytical cell and an ESA 5020 guard cell. Electrochemical detection was performed at 220 mV and 1.0 nA with the guard cell at 350 mV. All samples were analyzed in triplicate. The limit of detection for NE was 0.2 pg/5 µl. The chromatographic data were collected and analyzed with a PowerChrom system (AD Instruments, Castle Hill, NSW, Australia) and expressed as pg/ml of sample. Baseline values were defined as the average extracellular fluid NE concentration of the three samples collected before saline or LPS administration.
2.3. Stereotaxic injections
For intra-POA injections, isoflurane-anesthetized rats were mounted in a stereotaxic frame and the alpha-adrenergic receptor antagonist phentolamine (5 µg; 1.0 µl), (Sigma Chemical Co., St. Louis, MO) or saline (1.0 µl) was injected bilaterally into the POA with a Hamilton syringe (Model #62, Hamilton Co., Reno, NV) lowered at a 15° angle to a site 1.4 mm lateral and 0.3 mm caudal from bregma at a depth of 8.6 mm below the dural surface (Paxinos and Watson, 2005). For NTS injections, lidocaine (2%; 1.0 µl; Sigma Chemical Co., St. Louis, MO) or saline (1.0 µl) was injected through a cannula lowered vertically into the NTS 1.6 mm lateral and 13.3 mm caudal to bregma and a depth of 7.9 mm below the dural surface (Paxinos and Watson, 2005). For injections into the OVLT, lidocaine (2%; 1.0 µl) or saline (1.0 µl) was injected on the midline 0.6 mm rostral from bregma and a depth of 8.0 mm below the dural surface (Paxinos and Watson, 2005). For injections into the area postrema, lidocaine (2%; 1.0 µl) or saline (1.0 µl) was injected, on the midline 14.0 mm caudal to bregma and a depth of 7.5 mm below the dural surface (Paxinos and Watson, 2005).
Drugs were dissolved in 0.9 % saline containing 0.2% Chicago Sky Blue dye to mark the injection sites. Drug injections were delivered at a constant rate over a 1 min period. Two minutes after the stereotaxic injection of lidocaine, phentolamine or saline, rats were administered LPS (15 mg/kg) or saline (1 ml/kg) i.v. and arterial pressure and heart rate were recorded at 1 min intervals for 1 or 3 h. The 15 mg/kg LPS dose was selected based on evidence that it produces a prolonged hypotension and results in a mortality rate of at least 60% (Varga et al., 1998) in contrast to 1mg/kg i.v. LPS which causes little or no mortality (Rosengarten et al, 2007; Yilmaz et al., 2008a).
At the end of each experiment, each rat was sacrificed with an overdose of isoflurane, the brain was removed, frozen on dry ice and sections (50 µm) were cut with a microtome cryostat (Microm Model HM505E, Waldorf, Germany). Sections were mounted on slides, stained with eosin, air dried and cover slipped and the location of the cannula tip was confirmed. Only data from confirmed placement of the cannulas were included in this report.
2.4. Subdiaphragmatic vagotomy
The abdomen of isoflurane-anesthetized rats was opened by a midline incision and the esophagus and the stomach were gently retracted. The dorsal and ventral trunks of the vagus nerves around the esophagus were carefully dissected and cut with microsurgery scissors immediately below the diaphragm. Control animals underwent the same procedure except the nerves were not severed. Fifteen minutes later animals were administered LPS (15 mg/kg) or saline (1 ml/kg) i.v. and blood pressure and heart rate were monitored for 60 minutes.
2.5. Cervical vagotomy
Following a midline dissection of the cervical region, the left and right vagus nerves were separated carefully from neighboring tissues and cut simultaneously with microsurgery scissors. Control animals underwent the same procedure except the nerves were not severed. Fifteen minutes later animals were administered LPS (15 mg/kg) or saline (1 ml/kg) i.v. and blood pressure and heart rate were monitored for 60 minutes.
2.6. Statistical analysis
Data are presented as mean ± standard error of the mean (SEM) and were analyzed by repeated measure two-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test using SigmaStat 3.0 (SPSS, Chicago, IL, U.S.A.). A two sided P value of <0.05 was considered significant.
3. RESULTS
3.1. High Dose LPS increases extracellular fluid NE concentrations in the POA
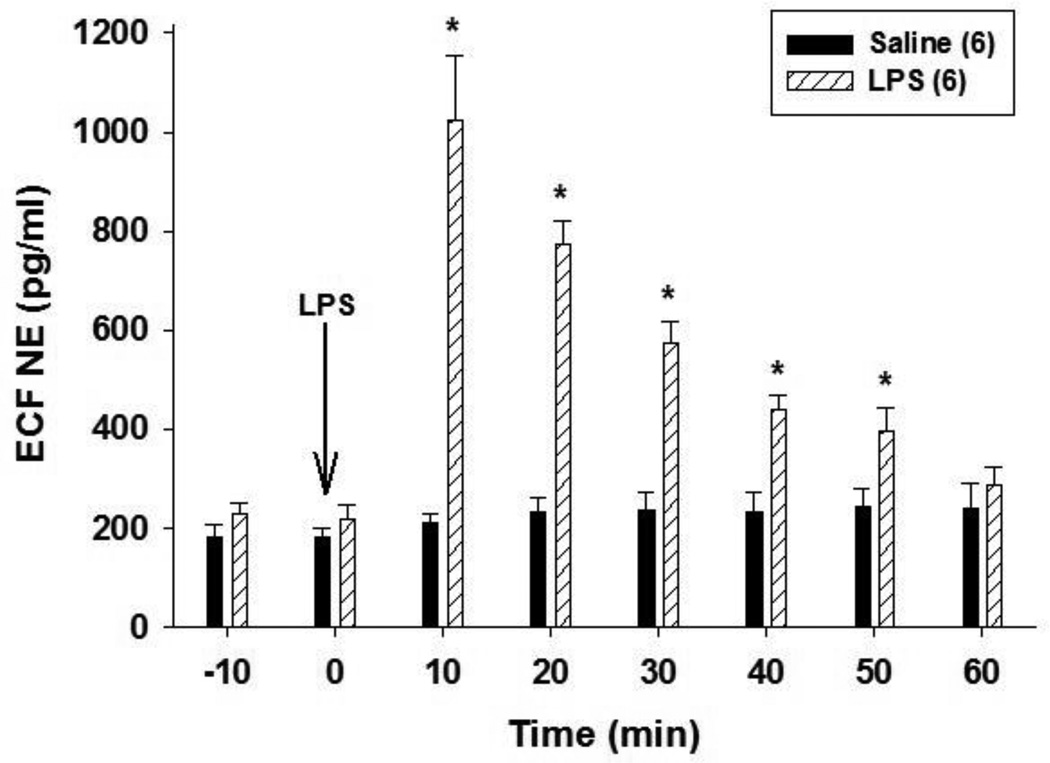
Previous studies showed that i.v. administration of 1 mg/kg LPS elevates extracellular NE concentrations in the POA although the effect of higher LPS doses has not been reported (Villanueva et al., 2009). To test this, isoflurane-anesthetized rats were treated with 15 mg/kg LPS i.v. and extracellular NE concentrations were sampled by microdialysis and analyzed by HPLC. Intravenous LPS administration increased extracellular NE concentrations in the POA significantly (F1,7 = 25.24; P < 0.001) (Fig. 1). Extracellular NE increased to approximately 500 % of control values within 10 min of LPS administration and remained significantly above control values for at least 50 min (Fig. 1). Saline administration did not affect extracellular fluid NE concentrations in the POA significantly (Fig. 1). These data show that 15 mg/kg LPS elevates extracellular NE in the POA as shown previously for a lower dose of LPS (Villanueva et al., 2009).
Figure 1.
Intravenous LPS administration increases extracellular fluid NE concentrations in the POA. Isoflurane-anesthetized rats were treated with LPS (15 mg/kg) or saline i.v. and extracellular NE concentrations were sampled 10 min before and at 10 min intervals thereafter. The numbers in parentheses indicate the number of animals in each group. Data represent the mean ± SEM and were analyzed using two-way repeated measures ANOVA followed by Tukey’s test. ECF = Extracellular fluid. *, P < 0.05, significantly different from extracellular NE concentrations at the same time point in saline treated animals.
3.2. Phentolamine injection into the POA inhibits LPS hypotension
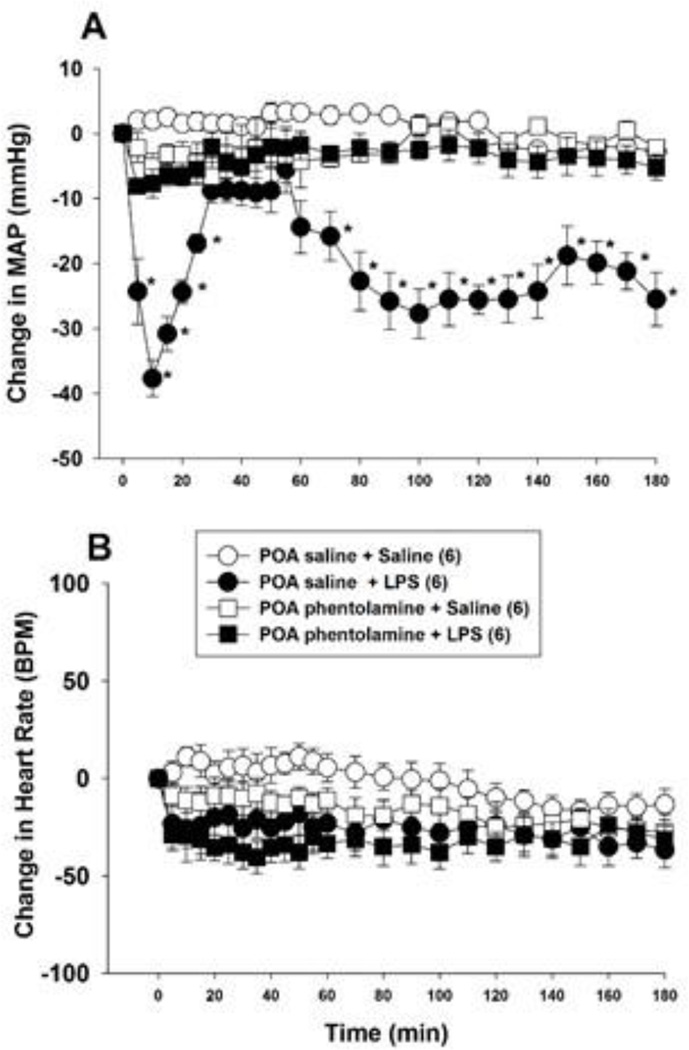
Intravenous administration of 15 mg/kg LPS produced a biphasic fall in arterial pressure (Fig. 2A). Mean arterial pressure began to decline within 5 min of LPS administration, reached its nadir at 10 min, and returned toward baseline values within 35 min (Fig. 2A). Arterial pressure began to decline a second time after 55 min and remained significantly below control values for the remainder of the 3 h experiment (Fig. 2A). Lipopolysaccharide administration did not affect heart rate significantly (Fig. 2B).
Figure 2.
Bilateral phentolamine injection into the POA inhibits the hypotension produced by i.v. LPS (15 mg/kg) injection. Phentolamine (5 µg/µl) or saline (1 µl) was injected into the POA of anesthetized rats bilaterally and, 2 min later, LPS or saline was administered i.v. Mean arterial pressure (MAP; panel A) and heart rate (panel B) were monitored for 180 min. The numbers in parentheses indicate the number of animals in each group. Baseline MAP and heart rate values were: POA saline + i.v. saline = 109.8 ± 4.2 mmHg and 361 ± 25 BPM; POA saline + i.v. LPS = 112.2 ± 3.7 mmHg and 371 ± 19 BPM; POA phentolamine + i.v. saline = 105.1 ± 2.9 mm Hg and 377 ± 31 BPM; POA phentolamine + i.v. LPS = 114.5 ± 4.1 mmHg and 325 ± 12 BPM. Data were analyzed using two-way repeated measures ANOVA followed by Tukey’s test. *, P < 0.05, significantly different from the POA saline plus i.v. saline treated group. BPM = beats per minute.
In an earlier study, we showed that bilateral phentolamine injection into the POA prevented the hypotension induced by a lower LPS dose, 1 mg/kg i.v. To test whether alpha-adrenergic receptors mediate the cardiovascular effects of 15 mg/kg LPS, we microinjected phentolamine (5 µg; 1 µl) or saline (1 µl) bilaterally into the POA before treating rats with 15 mg/kg LPS i.v. Figure 2A shows that phentolamine reduced the initial depressor effect of LPS and inhibited the second, decompensatory phase of LPS-induced hypotension significantly (F 3,24 = 12.23; P < 0.001) (Fig. 2A). Phentolamine did not affect arterial pressure significantly in saline treated control animals (Fig. 2A) and did not influence heart rate significantly in either control or LPS treated animals (Fig 2B).
3.3. Acute subdiaphragmatic vagotomy
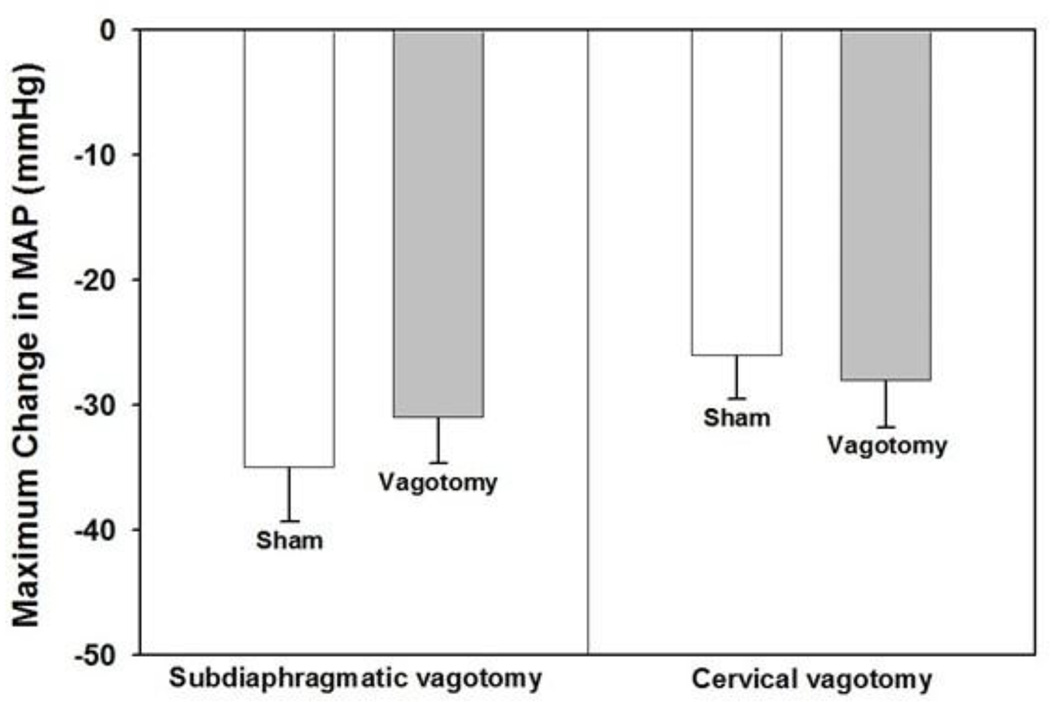
To test the hypothesis that vagus nerve afferents mediate the depressor response evoked by 15 mg/kg LPS we transected the vagus nerve below the diaphragm 15 min before injecting LPS i.v. Acute subdiaphragmatic vagotomy did not inhibit LPS hypotension significantly compared to sham vagotomy, however. Figure 3A shows that LPS administration lowered arterial pressure to approximately the same extent in vagotomized rats as it did in sham operated control animals. Heart rate was unaffected by LPS in both vagotomized and sham-operated animals (data not shown). Acute subdiaphragmatic vagotomy did not affect baseline arterial pressure (sham surgery = 109.7 ± 4.7 mmHg; vagotomy = 112.9 ± 3.3 mmHg) or heart rate (sham surgery = 388 ± 29 BPM; vagotomy = 357 ± 19 BPM) significantly. Previously, we showed that subdiaphragmatic vagotomy had no demonstrable effect on baseline arterial pressure or heart rate during the 60 min time course of the experiment (Yilmaz et al., 2008a).
Figure 3.
Neither subdiaphragmatic nor cervical vagotomy inhibit the hypotension evoked by LPS. Subdiaphragmatic or cervical vagotomy was performed 15 min before rats were administered LPS (15 mg/kg) or saline i.v. and mean arterial pressure (MAP) and heart rate were monitored for 60 min (n = animals in each group). Baseline MAP values for subdiaphragmatic vagotomy were: sham vagotomy + LPS = 110.5 ± 3.3 mmHg; vagotomy + LPS = 106.6 ± 3.2 mmHg. Baseline MAP for cervical vagotomy were: sham vagotomy + LPS = 121.5 ± 6.7 mmHg; vagotomy + LPS = 111.9 ± 5.8 mmHg. Data are presented as the mean ± SEM maximum change in MAP after LPS administration and were analyzed with two-way repeated measure ANOVA followed by Tukey’s test.
3.4. Cervical vagotomy
To test whether a higher level of vagotomy would inhibit the depressor response induced by 15 mg/kg LPS the vagus nerves were cut at the cervical level 15 min before LPS administration. Bilateral cervical vagotomy also failed to prevent LPS hypotension (Fig. 3B). Cervical vagotomy did not affect baseline mean arterial pressure or heart rate values compared to sham operated controls (data not shown). Together, these data show that neither subdiaphragmatic nor cervical vagotomy inhibits the hypotension produced by 15 mg/kg LPS.
3.5. Lidocaine injection into the NTS
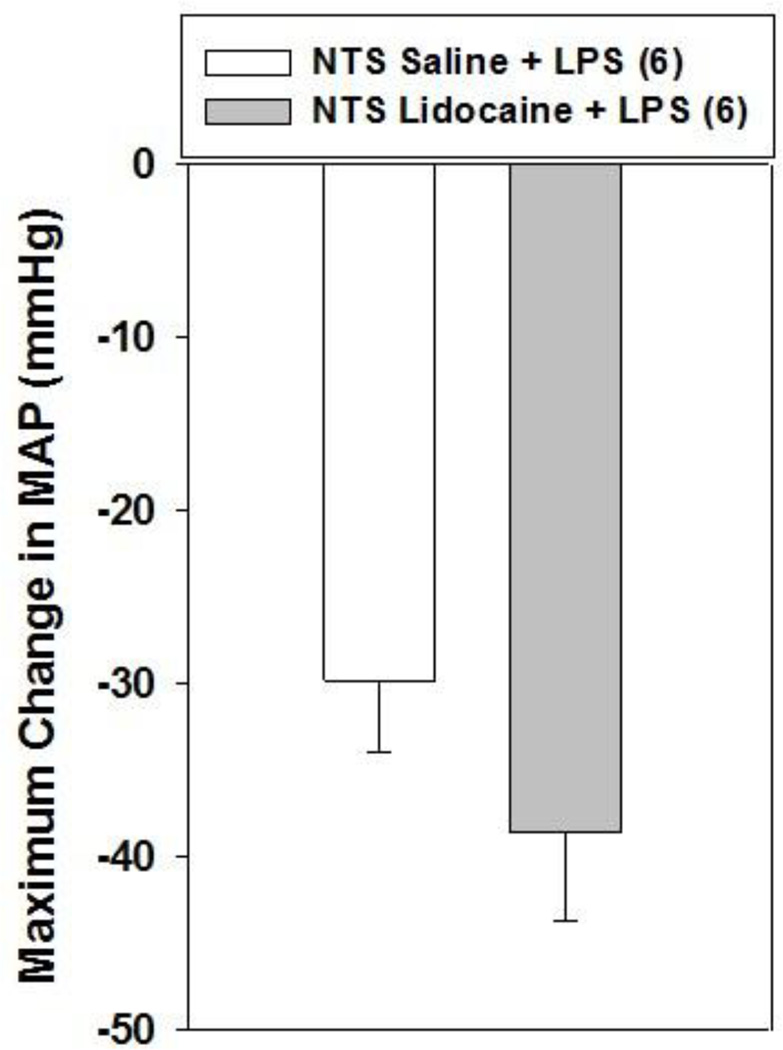
To further investigate whether vagus nerve afferents mediate the hypotension evoked by 15 mg/kg LPS we inhibited neuronal activity in the NTS with the local anesthetic lidocaine before treating rats with LPS. Lidocaine (2%; 1.0 µl) injection into the NTS failed to inhibit the hypotension evoked by 15 mg/kg LPS, however (Fig. 4). Lidocaine injection into the NTS had no effect on arterial pressure in control animals and did not influence heart rate in either control or LPS treated animals (data not shown). These results are in agreement with the results of acute vagotomy experiments and further support the hypothesis that 15 mg/kg LPS does not lower arterial pressure by activating vagus nerve afferents.
Figure 4.
Bilateral lidocaine injection into the NTS does not affect the hypotension produced by i.v. LPS (15 mg/kg) injection. Lidocaine (2%; 1 µl) or saline (1 µl) was injected bilaterally into the NTS of anesthetized rats and, 2 min later, LPS or saline was administered i.v. Mean arterial pressure (MAP) and heart rate were monitored for 60 min. The numbers in parentheses indicate the number of animals in each group. Baseline MAP values were: NTS saline + LPS = 113.3 ± 2.8 mmHg; NTS lidocaine + LPS = 117.2 ± 2.6 mmHg. Data are presented as the mean ± SEM maximum change in MAP after LPS administration and were analyzed with two-way repeated measures ANOVA followed by Tukey’s test.
3.6. Lidocaine injection into the OVLT or area postrema
Lipopolysaccharide raises body temperature through two different mechanisms depending on the dose; low hyperthermic LPS doses activate vagal nerve afferents whereas high doses activate receptors in circumventricular organs. This raises the possibility that a high hypotensive dose of LPS may lower arterial pressure through a similar mechanism involving the OVLT and/or area postrema.
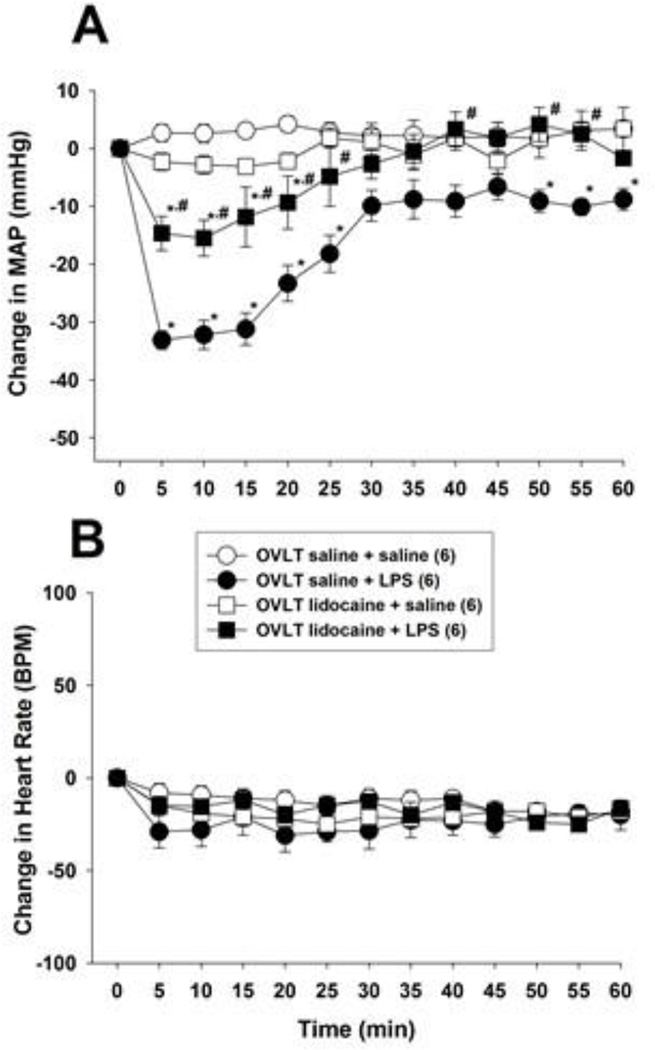
To test this hypothesis we injected lidocaine into the OVLT before treating rats with LPS. Once again i.v. administration of 15 mg/kg LPS decreased arterial pressure within 5 min in saline treated control animals but did not affect heart rate significantly (Fig. 5). Microinjection of lidocaine (2%; 1.0 µl) into the OVLT inhibited the fall in arterial pressure evoked by 15 mg/kg i.v. LPS significantly (F 3,36 = 6.62; P < 0.001). Lidocaine had no significant effect on mean arterial pressure or heart rate in control animals treated with i.v. saline in lieu of LPS. These data indicate that activation of neurons within the OVLT is necessary for 15 mg/kg LPS to lower arterial pressure.
Figure 5.
Lidocaine injection into the OVLT inhibits the hypotension produced by i.v. LPS (15 mg/kg) injection. Lidocaine (2%; 1 µl) or saline (1 µl) was injected into the OVLT of anesthetized rats and, 2 min later, LPS or saline was administered i.v. and mean arterial pressure (MAP; panel A) and heart rate (panel B) were monitored for 60 min. The numbers in parentheses indicate the number of animals in each group. Baseline MAP and heart rate values were: OVLT saline + i.v. saline = 109.8 ± 4.2 mmHg and 361 ± 25 BPM; OVLT saline + i.v. LPS = 112.2 ± 3.7 mmHg and 371 ± 19 BPM; OVLT lidocaine + i.v. saline = 105.1 ± 2.9 mm Hg and 377 ± 31 BPM; OVLT lidocaine + i.v. LPS = 114.5 ± 4.1 mmHg and 325 ± 12 BPM. Data were analyzed using two-way repeated measures ANOVA followed by Tukey’s test. *, P < 0.05, significantly different from the OVLT saline plus i.v. saline treated group. #, P < 0.05, significantly different from the OVLT saline plus i.v. LPS treated group. BPM = beats per minute.
In subsequent experiments, we tested whether the area postrema was involved in the response to 15 mg/kg i.v. LPS. Lidocaine administration into area postrema failed to inhibit the depressor response to LPS. The maximum fall in mean arterial pressure evoked by LPS following lidocaine injection into the area postrema (−35.4 ± 4.4 mmHg; n = 5) was not significantly different from the maximum change following saline injection (−39.2 ± 6.6 mmHg; n = 5). Heart rate was not affected significantly by lidocaine, LPS or combined treatment (data not shown). Together these results show that inhibition of neuronal activity in the OVLT, but not the area postrema, attenuates the depressor response evoked by 15 mg/kg LPS.
3.7. Confirmation of injection sites
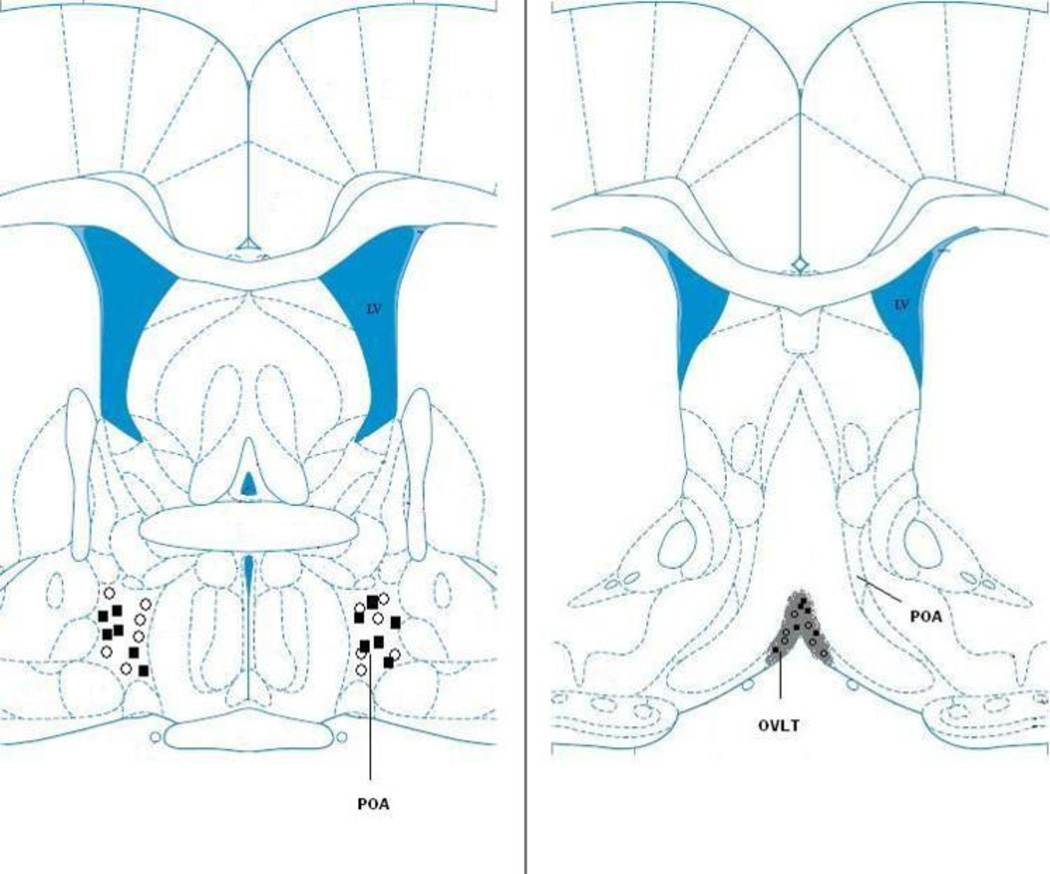
Figure 6 illustrates the placement of phentolamine and saline injections targeted on the preoptic area (Fig. 6; left panel) and lidocaine and saline injections into the OVLT (Fig. 6; right panel).
Figure 6.
Schematic representation of the location of microinjections into the POA and OVLT. Left panel: The open circles (○) and filled squares (■) indicate the location of phentolamine injection sites in saline and LPS treated animals, respectively. Right panel: The open circles (○) and filled squares (■) indicate the location of lidocaine injections in saline and LPS treated animals, respectively.
4. DISCUSSION
In this report we show that the initial fall in arterial pressure evoked by a relatively high dose of LPS, 15 mg/kg i.v., is attenuated by inhibiting neuronal activity in the OVLT with lidocaine but is unaffected by either cervical or subdiaphragmatic vagotomy or by lidocaine injection into the NTS. These findings contrast the response to 1 mg/kg i.v. LPS which is blocked by vagotomy and intra-NTS lidocaine injection but not by inactivation of the OVLT or area postrema (Yilmaz et al., 2008a). The effects of both 1 mg/kg and 15 mg/kg LPS appear to be mediated by NE release in the POA because both doses elevated extracellular NE concentrations in the POA (Villanueva et al., 2009) and because phentolamine injection into the POA prevented LPS hypotension irrespective of dose (Yilmaz et al., 2008a). Together, these data indicate that LPS lowers arterial pressure through two different anatomical sites, depending on the dose, which converge on NE neurons within the POA.
The OVLT is in relatively close proximity to the POA, raising concern that the inhibitory effect of lidocaine could result from spread of the lidocaine injectate from the OVLT into the POA. Indeed, microinjection of lidocaine directly into the POA blocks LPS (1 mg/kg i.v.) hypotension completely (Yilmaz et al. 2008b). An indirect effect seems unlikely, however, because a 1 µl lidocaine injection inhibits neuronal activity in an effective radius of 0.8 mm (Sandkühler et al., 1987), slightly less than the distance between the OVLT and POA injections used in this and previous studies (Yilmaz et al., 2008a). Moreover, in earlier experiments, lidocaine injection into the OVLT had no effect whatsoever on the hypotension evoked by 1 mg/kg LPS (Yilmaz et al., 2008a). This observation argues against the possibility that lidocaine prevents the cardiovascular effects of a higher dose of LPS, 15 mg/kg, by spreading from the OVLT into, and inactivating, the POA.
The present finding that vagotomy fails to prevent the hypotension caused by 15 mg/kg LPS is consistent with an earlier report by Pitterman et al. (1983) that abdominal vagotomy was unable to prevent the fall in arterial pressure evoked 100 mg/kg i.v. LPS in conscious rats and failed to reduce mortality caused by this supramaximal LPS dose. On the other hand, Mailman (2002) reported that the depressor effect of 5 mg/kg i.v. LPS was blocked completely by application of lidocaine to the subdiaphragmatic vagus of pentobarbital-anesthetized rats, consistent with the finding that vagotomy inhibits the response to 1 mg/kg LPS reported earlier (Yilmaz et al., 2008a). Together, these data suggest that the ‘cut off’ for vagal mediation of LPS hypotension is between 5 and 15 mg/kg LPS.
These experiments were predicated on extensive evidence that the mechanism responsible for the thermoregulatory effects of LPS and cytokines is dose-dependent: very low LPS doses (1 µg/kg i.v.) cause fever by activating vagus nerve afferents whereas higher doses (30 µg/kg i.v.) activate receptors in the OVLT, area postrema (AP) and, perhaps, other circumventricular organs (Sehic and Blatteis, 1996; Romanovsky et al., 1997; Hansen et al., 2001; Blatteis et al., 2005; Romanovsky et al., 2005). The vagus nerve is also thought to mediate some of the behavioral and endocrine effects of LPS. The behavioral depression and reduced social interaction caused by relatively low doses of LPS (100 µg/kg to 1.25 mg/kg i.p.) are prevented by vagotomy (Bluthé et al., 1994; Konsman et al., 2000) or NTS inactivation with bupivacaine (Marvel et al., 2004), for example. Interestingly, however, Turek et al. (2005) reported that vagotomy did not interfere with the ability of rats to select a warm ambient temperature when placed in a thermal environmental gradient after receiving LPS (50 µg/kg LPS i.p.). These data suggest that behavioral depression and thermoregulatory behavior may be mediated by different central pathways.
Subdiaphragmatic vagotomy also attenuates the hyperalgesia (Watkins et al., 1994) and elevated circulating ACTH concentrations LPS provokes (Gaykema et al., 1995; Wieczorek, 2005). Curiously, however, vagotomy does not prevent the rise in circulating corticosterone caused by LPS in rats (Gaykema et al., 1995; Hansen et al., 2000) although, evidently, it does so in mice (Wieczorek et al., 2005). Collectively, these data indicate that the vagus nerve conveys the ‘signal’ from circulating LPS to the brain for some, although not all, LPS responses. A role for the OVLT, if any, in mediating these responses has not been extensively evaluated. Indeed, Konsman et al. (2000) proposed that vagal nerve afferents mediate the behavioral and endocrine effects of LPS that comprise sickness behavior whereas the OVLT mediates LPS fever at behaviorally relevant LPS doses (250 µg/kg, i.p.).
Nonetheless, there is abundant evidence that the OVLT and adjacent regions of the anteroventral third ventricle region (AV3V) are involved in cardiovascular regulation (McKinley et al., 2001). In transneuronal retrograde tracing experiments, injection of a pseudorabies viral vector into sympathetic ganglia labeled neurons in the POA and, after an additional time delay, the OVLT and adjacent medial preoptic area indicating that both the OVLT and POA influence sympathetic activity transynaptically (Westerhaus and Loewy, 1999). Data from track tracing studies further indicate that the OVLT sends direct projections to the lateral, medial and ventrolateral preoptic areas (Camacho and Phillips, 1981; Chou et al., 2002; Uschakov et al., 2007), POA regions likely to be affected by the phentolamine and lidocaine injections used in this and an earlier study to prevent LPS hypotension (Yilmaz et al., 2008a; 2008b). Electrical stimulation of the AV3V region lowers arterial pressure (Knuepfer et al., 1984) although selective stimulation of the OVLT produces the opposite response; it elevates blood pressure by causing vasoconstriction in renal and mesenteric vascular beds (Mangiapane and Brody, 1987). Electrical stimulation of the adjacent median preoptic nucleus produces hypotension and hindquarter vasodilation, which presumably explains the depressor effect of AV3V stimulation (Mangiapane and Brody, 1987). These physiological studies argue against the conclusion that LPS lowers arterial pressure by activating the OVLT and suggest, instead, that the adjacent median preoptic nucleus could mediate the response.
Nevertheless, electrical stimulation, however precisely localized, is a rather non-selective means of reproducing the pharmacological effects of LPS. Other lines of evidence support the conclusion that OVLT neurons mediate the depressor responses reported here. Ott et al. (2010) showed that LPS directly activates neurons and glial cells isolated from the OVLT of neonatal rats, causing rapid, transient increases in intracellular calcium concentrations in a subpopulation of neurons, astrocytes and microglia. This finding is consistent with evidence that Toll-like receptors (TLRs), including the TLR-4 receptor thought to mediate the effects of LPS, have been localized in the OVLT (Laflamme and Rivest, 2001) and are expressed by microglial cells (Olson and Miller, 2004). The rise in intracellular calcium concentrations LPS produces in vitro is consistent with evidence that LPS administration into the OVLT induces nitric oxide synthase (NOS) in vivo (Lin and Lin, 2000). Moreover, microinjection of hydroxylamine and other nitric oxide donors into the OVLT, to stimulate nitric oxide synthesis, lowers arterial pressure substantially, by 55 mm Hg, in urethane-anesthetized rats; conversely, NOS inhibitors increase blood pressure significantly (Lin et al., 1999). These data raise the possibility that LPS lowers arterial pressure by stimulating NO synthesis in the OVLT.
These considerations support the conclusion that LPS lowers arterial pressure by activating receptors in the OVLT which ultimately stimulates NE release in the POA. Anatomical tract tracing studies support a role for the POA in cardiovascular regulation, showing that POA neurons innervate the nucleus tractus solitarius, parabrachial nucleus, midbrain periaqueductal gray (PAG) and other cardioregulatory brain regions (Rizvi et al., 1992; Simerly and Swanson, 1988). Electrical or chemical stimulation of the POA lowers arterial pressure and induces c-fos expression in the ventrolateral column of the PAG (vlPAG), suggesting that the vlPAG mediates the response (Inui et al., 1995; Behbehani and Da Costa Gomez, 1996). Moreover, lidocaine injection into these fos immunoractive sites in the vlPAG inhibit the hypotensive response evoked by POA stimulation (Behbehani and Da Costa Gomez, 1996), as does destruction of cell bodies in the vlPAG with excitatory amino acid injections (Inui et al., 1995). Activation of vlPAG neurons is thought to influence cardiovascular function through a descending pathway that involves the midline raphe nuclei and ventrolateral medulla (Bago and Dean, 2001; Vagg et al., 2008). In preliminary studies, we found that lidocaine injection into the vlPAG prevented the hypotension evoked by LPS (1 mg/kg) suggesting that this previously characterized pathway from the POA to the vlPAG, midbrain raphe nuclei and ventrolateral medulla may mediate the response.
The mechanisms responsible for the rather unusual dose-related effects of LPS remain somewhat unclear. The simple observation that low hypotensive doses of LPS lower arterial pressure by activating vagal nerve afferents whereas higher doses act through the OVLT suggest that receptors and/or second messenger systems in the OVLT may exhibit a lower affinity for LPS than those in the vagus. Exactly how a similar dichotomous dose-response for fever can operate at doses that are orders of magnitude lower remains mechanistically enigmatic, however. Conceivably, LPS may evoke fever in the OVLT indirectly, by stimulating the release of cytokines, and could lower arterial pressure by directly activating TL-4 receptors on OVLT neurons (Ott et al., 2010) although this is, obviously, mere speculation. Nonetheless, despite these mechanistic ambiguities, the global implications of these findings remain noteworthy; evidence that endotoxic hypotension is initiated through a central mechanism may provide new avenues for treating or preventing septic shock.
Highlights.
At low doses LPS initially lowers arterial pressure by activating vagal afferents
High dose LPS initiates hypotension by activating the OVLT, but not the vagus
LPS lowers arterial pressure by raising extracellular NE concentrations in the POA
Phentolamine injection into the POA prevents LPS hypotension
ACKNOWLEDGEMENTS
This research was funded by a grant from the National Institute of Allergy and Infectious Disease (R15AI072744) awarded to Dr. Carlos Feleder.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Angus DC, van der Poll T. Severe sepsis and septic shock. N. Engl. J. Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- Azab AN, Kaplanski J. Vagotomy attenuates the effect of lipopolysaccharide on body temperature of rats in a dose-dependent manner. J. Endotoxin Res. 2001;7:359–364. [PubMed] [Google Scholar]
- Bago M, Dean C. Sympathoinhibition from ventrolateral periaqueductal gray mediated by 5-HT1A receptors in the RVLM. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R976–R984. doi: 10.1152/ajpregu.2001.280.4.R976. [DOI] [PubMed] [Google Scholar]
- Behbehani MM, Da Costa Gomez TM. Properties of a projection pathway from the medial preoptic nucleus to the midbrain periaqueductal gray of the rat and its role in the regulation of cardiovascular function. Brain Res. 1996;740:141–150. doi: 10.1016/s0006-8993(96)00858-x. [DOI] [PubMed] [Google Scholar]
- Blatteis CM, Li S, Li Z, Feleder C, Perlik V. Cytokines, PGE2 and endotoxic fever: a re-assessment. Prostaglandins Other Lipid Mediat. 2005;76:1–18. doi: 10.1016/j.prostaglandins.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Bluthé RM, Walter V, Parnet P, Layé S, Lestage J, Verrier D, Poole S, Stenning BE, Kelley KW, Dantzer R. Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. C. R. Acad. Sci. III. 1994;317:499–503. [PubMed] [Google Scholar]
- Bosmann M, Ward PA. The inflammatory response in sepsis. Trends Immunol. 2013;34:129–136. doi: 10.1016/j.it.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho A, Phillips MI. Horseradish peroxidase study in rat of the neural connections of the organum vasculosum of the lamina terminalis. Neurosci. Lett. 1981;25:201–204. doi: 10.1016/0304-3940(81)90391-8. [DOI] [PubMed] [Google Scholar]
- Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB. Afferents to the ventrolateral preoptic nucleus. J. Neurosci. 2002;22:977–990. doi: 10.1523/JNEUROSCI.22-03-00977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaykema RP, Dijkstra I, Tilders FJ. Subdiaphragmatic vagotomy suppresses endotoxin-induced activation of hypothalamic corticotropin-releasing hormone neurons and ACTH secretion. Endocrinology. 1995;136:4717–4720. doi: 10.1210/endo.136.10.7664696. [DOI] [PubMed] [Google Scholar]
- Hansen MK, Nguyen KT, Fleshner M, Goehler LE, Gaykema RP, Maier SF, Watkins LR. Effects of vagotomy on serum endotoxin, cytokines, and corticosterone after intraperitoneal lipopolysaccharide. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;278:R331–R336. doi: 10.1152/ajpregu.2000.278.2.R331. [DOI] [PubMed] [Google Scholar]
- Hansen MK, O’Connor KA, Goehler LE, Watkins LR, Maier SF. The contribution of the vagus nerve in interleukin-lβ-induced fever is dependent on dose. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R929–R934. doi: 10.1152/ajpregu.2001.280.4.R929. [DOI] [PubMed] [Google Scholar]
- Inui K, Nomura J, Murase S, Nosaka S. Facilitation of the arterial baroreflex by the preoptic area in anaesthetized rats. J. Physiol. 1995;488:521–531. doi: 10.1113/jphysiol.1995.sp020987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawad I, Lukšić I, Rafnsson SB. Assessing available information on the burden of sepsis: global estimates of incidence, prevalence and mortality. J. Glob. Health. 2012;2:010404. doi: 10.7189/jogh.02.010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King EG, Bauzá GJ, Mella JR, Remick DG. Pathophysiologic mechanisms in septic shock. Lab. Invest. 2014;94:4–12. doi: 10.1038/labinvest.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuepfer MM, Johnson AK, Brody MJ. Vasomotor projections from the anteroventral third ventricle (AV3V) region. Am. J. Physiol. Heart Circ. Physiol. 1984;247:H139–H145. doi: 10.1152/ajpheart.1984.247.1.H139. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Luheshi GN, Bluthé RM, Dantzer R. The vagus nerve mediates behavioral depression, but not fever, in response to peripheral immune signals; a functional anatomical analysis. Eur. J. Neurosi. 2000;12:4434–4446. doi: 10.1046/j.0953-816x.2000.01319.x. [DOI] [PubMed] [Google Scholar]
- Laflamme N, Rivest S. Toll-like receptor 4: the missing link of the cerebral innate immune response triggered by circulating gram-negative bacterial cell wall components. FASEB J. 2001;15:155–163. doi: 10.1096/fj.00-0339com. [DOI] [PubMed] [Google Scholar]
- Lin MT, Lin JH. Involvement of tyrosine kinase in the pyrogenic fever exerted by NOS pathways in organum vasculosum laminae terminalis. Neuropharmacology. 2000;39:347–352. doi: 10.1016/s0028-3908(99)00127-6. [DOI] [PubMed] [Google Scholar]
- Lin MT, Pan SP, Lin JH, Yang YL. Central control of blood pressure by nitrergic mechanisms in organum vasculosum laminae terminalis of rat brain. Br. J. Pharmacol. 1999;127:1511–1517. doi: 10.1038/sj.bjp.0702699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailman D. A role for abdominal vagal afferents in lipopolysaccharide-induced hypotension. Shock. 2002;18:177–181. doi: 10.1097/00024382-200208000-00015. [DOI] [PubMed] [Google Scholar]
- Mangiapane ML, Brody MJ. Vasoconstrictor and vasodilator sites within anteroventral third ventricle region. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1987;253:R827–R831. doi: 10.1152/ajpregu.1987.253.6.R827. [DOI] [PubMed] [Google Scholar]
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- Marvel FA, Chen CC, Badr N, Gaykema RPA, Goehler LE. Reversible inactivation of the dorsal vagal complex blocks lipopolysaccharide-induced social withdrawal and c-Fos expression in central autonomic nuclei. Brain Behav. Immun. 2004;18:123–134. doi: 10.1016/j.bbi.2003.09.004. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Allen AM, May CN, McAllen RM, Oldfield BJ, Sly D, Mendelsohn FAO. Neural pathways from the lamina terminalis influencing cardiovascular and body fluid homeostasis. Clin. Exp. Pharmacol. Physiol. 2001;28:990–992. doi: 10.1046/j.1440-1681.2001.03592.x. [DOI] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J. Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Ott D, Murgott J, Rafalzik S, Wuchert F, Schmalenbeck B, Roth J, Gerstberger R. Neurons and glial cells of the rat organum vasculosum laminae terminalis directly respond to lipopolysaccharide and pyrogenic cytokines. Brain Res. 2010;1363:93–106. doi: 10.1016/j.brainres.2010.09.083. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 2005. [Google Scholar]
- Pitterman AB, Friedlander G, Kelly G, Ropchak TG, Goldstein DJ, Keiser HR. Abdominal vagotomy does not modify endotoxic shock in rats. Life Sci. 1983;33:1033–1037. doi: 10.1016/0024-3205(83)90657-4. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Shipley MT. Reciprocal connections between the medial preoptic area and the midbrain periaqueductal gray in rat: a WGA-HRP and PHA-L study. J. Comp. Neurol. 1992;315:1–15. doi: 10.1002/cne.903150102. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Almeida MC, Aronoff DM, Ivanov AI, Konsman JP, Steiner A, Turek VF. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front. Biosci. 2005;10:2193–2216. doi: 10.2741/1690. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Simons CT, Székely M, Kulchitsky VA. The vagus nerve in the thermoregulatory response to systemic inflammation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1997;273:R407–R413. doi: 10.1152/ajpregu.1997.273.1.R407. [DOI] [PubMed] [Google Scholar]
- Rosengarten B, Hecht M, Auch D, Ghofrani HA, Schermuly RT, Grimminger F, Kaps M. Microcirculatory dysfunction in the brain precedes changes in evoked potentials in endotoxin-induced sepsis syndrome in rats. Cerebrovasc. Dis. 2007;23:140–147. doi: 10.1159/000097051. [DOI] [PubMed] [Google Scholar]
- Sandkühler J, Maisch B, Zimmermann M. The use of local anaesthetic microinjections to identify central pathways: a quantitative evaluation of the time course and extent of the neuronal block. Exp. Brain Res. 1987;68:168–178. doi: 10.1007/BF00255242. [DOI] [PubMed] [Google Scholar]
- Sehic E, Blatteis CM. Blockade of lipopolysaccharide-induced fever by subdiaphragmatic vagotomy in guinea pigs. Brain Res. 1996;726:160–166. [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: A Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J. Comp. Neurol. 1988;270:209–242. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- Steiner AA, Hunter JC, Phipps SM, Nucci TB, Oliveira DL, Roberts JL, Scheck AC, Simmons DL, Romanovsky AA. Cyclooxygenase-1 or −2 -- which one mediates lipopolysaccharide-induced hypothermia? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:R485–R494. doi: 10.1152/ajpregu.91026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek VF, Olster DH, Ettenberg A, Carlisle HJ. The behavioral thermoregulatory response of febrile female rats is not attenuated by vagotomy. Pharmacol. Biochem. Behav. 2005;80:115–121. doi: 10.1016/j.pbb.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Ulloa L, Tracey KJ. The ‘cytokine’ profile: a code for sepsis. Trends Mol. Med. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Uschakov A, Gong H, McGinty D, Szymusiak R. Efferent projections from the median preoptic nucleus to sleep- and arousal regulatory nuclei in the rat brain. Neuroscience. 2007;150:104–120. doi: 10.1016/j.neuroscience.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagg DJ, Bandler R, Keay KA. Hypovolemic shock: Critical involvement of a projection from the ventrolateral periaqueductal gray to the caudal midline medulla. Neuroscience. 2008;152:1099–1109. doi: 10.1016/j.neuroscience.2007.10.070. [DOI] [PubMed] [Google Scholar]
- Van Amersfoort ES, Van Berkel TJC, Kuiper J. Receptors, mediators and mechanisms involved in bacterial sepsis and septic shock. Clin. Microbiol. Rev. 2003;16:379–414. doi: 10.1128/CMR.16.3.379-414.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga K, Wagner JA, Bridgen DT, Kunos G. Platelet- and macrophage-derived endogenous cannabinoids are involved in endotoxin-induced hypotension. FASEB J. 1998;12:1035–1044. doi: 10.1096/fasebj.12.11.1035. [DOI] [PubMed] [Google Scholar]
- Villanueva A, Yilmaz MS, Millington WR, Cutrera RA, Stouffer DG, Parsons LH, Cheer JF, Feleder C. Central cannabinoid 1 receptor antagonist administration prevents endotoxic hypotension affecting norepinephrine release in the preoptic anterior hypothalamic area. Shock. 2009;32:614–620. doi: 10.1097/SHK.0b013e3181a4fd8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL. Clinical sepsis and septic shock - definition, diagnosis and management principles. Langenbecks Arch. Surg. 2008;393:817–824. doi: 10.1007/s00423-008-0343-1. [DOI] [PubMed] [Google Scholar]
- Vincent JL, De Backer D. Circulatory shock. N. Engl. J. Med. 2013;369:1726–1734. doi: 10.1056/NEJMra1208943. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Wiertelak EP, Goehler LE, Mooney-Heiberger K, Martinez J, Furness L, Smith KP, Maier SF. Neurocircuitry of illness-induced hyperalgesia. Brain Res. 1994;639:283–299. doi: 10.1016/0006-8993(94)91742-6. [DOI] [PubMed] [Google Scholar]
- Westerhaus MJ, Loewy AD. Sympathetic-related neurons in the preoptic region of the rat identified by viral transneuronal labeling. J. Comp. Neurol. 1999;414:361–378. [PubMed] [Google Scholar]
- Wieczorek M, Swiergiel AH, Pournajafi-Nazarloo H, Dunn AJ. Physiological and behavioral responses to interleukin-1β and LPS in vagotomized mice. Physiol. Behav. 2005;85:500–511. doi: 10.1016/j.physbeh.2005.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz MS, Göktalay G, Millington WR, Myer BS, Cutrera RA, Feleder C. Lipopolysaccharide-induced hypotension is mediated by a neural pathway involving the vagus nerve, the nucleus tractus solitarius and alpha-adrenergic receptors in the preoptic anterior hypothalamic area. J. Neuroimmunol. 2008a;203:39–49. doi: 10.1016/j.jneuroim.2008.06.023. [DOI] [PubMed] [Google Scholar]
- Yilmaz MS, Millington WR, Feleder C. The preoptic anterior hypothalamic area mediates initiation of the hypotensive response induced by LPS in male rats. Shock. 2008b;29:232–237. doi: 10.1097/shk.0b013e3180caac7e. [DOI] [PubMed] [Google Scholar]