Abstract
Rationale
Post-traumatic stress disorder (PTSD) and alcoholism are frequently co-morbid, suggesting the possibility of overlapping neural substrates. The neurokinin 1 (NK1) receptor for Substance P (SP) has been implicated in both stress- and alcohol-related behaviors. The NK1 antagonist aprepitant, clinically available as a treatment for chemotherapy-induced nausea, offers a tool to probe a potential role of the SP/NK1 system in co-morbid PTSD and alcoholism.
Objectives
To evaluate the efficacy of aprepitant for treatment of co-morbid PTSD and alcoholism.
Methods
Fifty-three patients with PTSD and alcoholism were admitted for 4 weeks to an inpatient unit at the NIH Clinical Center, and randomized to double-blind aprepitant (125 mg/day; based on PET studies reporting >90% central receptor occupancy at this dose) or placebo. After reaching steady state, subjects were assessed for PTSD symptom severity; behavioral and neuroendocrine responses to stress and alcohol cues; and fMRI responses to stimuli with positive or negative emotional valence.
Results
Aprepitant treatment had no effect on PTSD symptoms or subjective or physiological responses to stress or alcohol cues. However, aprepitant robustly potentiated ventromedial prefrontal cortex (mPFC) fMRI responses to aversive visual stimuli.
Conclusions
Despite the lack of effect on PTSD symptoms and responses to stress/alcohol cues, NK1 antagonism activated the ventral mPFC, an area considered hypoactive in PTSD, during exposure to aversive stimuli. Because this brain area is critically important for extinction of fear memories and in alcohol craving and relapse, our finding suggests that NK1 antagonism might be a useful pharmacological treatment to enhance extinction-based cue-exposure therapies.
Clinical trials registration number
Keywords: alcohol dependence, stress, anxiety, PTSD, NK1, craving
INTRODUCTION
Posttraumatic stress disorder (PTSD) is associated with extensive psychiatric co-morbidity, and about 50% of individuals diagnosed with PTSD also have a diagnosis of an alcohol use disorder (Kessler et al., 1995). The mechanisms underlying this co-morbidity remain unclear, but both conditions are associated with high levels of stress reactivity. Recently, a behavioral intervention has demonstrated efficacy in reduction of PTSD symptoms in this population (Mills et al., 2012), without similar improvement substance dependence symptomatology. Additional treatment options, particularly pharmacotherapies, are needed, as per the Institute of Medicine (2008).
Literature suggests that PTSD is maintained by changes in circuits regulating memory function following traumatic stress, including connections between the amygdala and medial prefrontal cortex (mPFC); these changes comprise hyperactivity of the amygdala and hypoactivity of the mPFC [reviewed in (Liberzon and Sripada, 2008)], as well as decreased volume of the anterior cingulate cortex (ACC), a part of the mPFC (Woodward et al., 2006). It has been proposed that the mPFC plays a role in the “contextualization” of stimuli, and that dysregulation of contextualization processes might drive PTSD symptoms. Relatedly, as reviewed by Acheson et al., (2012), preclinical and clinical data suggest that hippocampal dysfunction related to an inability to appropriately contextualize fear responses may be an etiological contributor to PTSD. Individuals with PTSD are more reactive to environmental stimuli, less able to inhibit responses to stress, and show impaired fear extinction than those without PTSD.
Stress also plays an important role in the development, maintenance, and relapse processes of alcoholism (Sinha et al., 2011a). Up-regulated amygdala function promotes escalation of voluntary alcohol consumption and stress-induced relapse to alcohol seeking. Further, research suggests an additive effect of trauma and alcohol cues on alcohol craving (Coffey et al., 2010). The interaction between PTSD and alcohol consumption is clinically important, as individuals with co-morbid PTSD and alcoholism show less improvement during treatment, relapse more rapidly to heavy drinking, and have more drinking days post-treatment than alcoholics without PTSD (Coffey et al., 2006).
Moreover, craving for alcohol predicts relapse (Gillespie et al., 2009; Lovallo et al., 2000; Santa Ana et al., 2006), and both alcohol cues and stress represent potent triggers for craving and relapse in alcohol dependent individuals (Adinoff et al., 2005; Cooney et al., 1997; Higley et al., 2011, Sinha et al., 2009). Studies using the cue reactivity (CR) paradigm have found differences between alcoholics and non-alcoholics in the response to alcohol cues, such that alcoholics show greater physiological and subjective responses to alcohol than do non-alcoholics (Monti et al., 1987). Moreover, this increased reactivity has been associated with subsequent drinking outcomes, at least among males (Rohsenow et al., 1994). Sinha and colleagues (2009) showed that abstinent alcoholics had greater craving for alcohol, subjective distress, negative emotionality, and blood pressure compared to social drinkers in response to both stress and cue scripts, although their cortisol and heart-rate responses were relatively blunted. Additional studies have also demonstrated increases in craving and stress in response to the stress and cue scripts among treatment-seeking alcoholics (Fox et al., 2007).
The neurokinin 1 (NK1) receptor, the preferred receptor for Substance P (SP) in the human brain, may offer a treatment target in alcoholics with PTSD. NK1 receptors are expressed in the amygdala, hippocampus and other components of neural stress-response circuitry. In experimental animals and humans, exposure to stressors causes a release of SP in the amygdala, while selective antagonism of NK1 receptors blocks behavioral stress responses (Schank et al., 2012). NK1 antagonists including aprepitant, a selective high-affinity antagonist of human NK1 receptors (Frank and Hargreaves, 2003), have been in development for mood and anxiety disorders, with mixed efficacy for treating depression (Keller et al., 2006; Kramer et al., 1998). Kramer and colleagues (1998) found an anti-depressant effect of aprepitant (then known as MK-869); this effect was not replicated by Keller et al. (2006).
NK1 antagonism may be particularly attractive for treating co-morbid PTSD and alcoholism because patients with PTSD have elevated basal levels of SP in cerebrospinal fluid, and because these levels are further elevated by presentation of trauma-associated cues (Geracioti et al., 2006). NK1 antagonism attenuates stress reactivity and decreases both alcohol craving in anxious alcoholics (George et al., 2008) and stress-induced reinstatement of alcohol seeking in a rat relapse model (Schank et al., 2011). Here, we carried a double-blind, placebo-controlled study to evaluate the effects of aprepitant in patients with co-morbid PTSD and alcoholism.
MATERIALS AND METHODS
Participants were recruited between January 2010 and January 2012 through advertisements in local media. Following phone screening, subjects were admitted to the NIH Clinical Center in Bethesda, MD, and underwent medically managed withdrawal if needed. Once they had an undetectable breath alcohol concentration and did not require benzodiazepines for withdrawal, they were evaluated for eligibility; eligibility determination typically took between five and seven days to complete. Detailed eligibility criteria are at http://www.clinicaltrials.gov/ct2/show/NCT00896038. In brief, subjects were 53 individuals between 21 – 50 years old, diagnosed with alcoholism and PTSD according to the Structured Clinical Interview for DSM-IV [SCID; (First et al., 1995)], and in good physical health. They were excluded if they presented with complicated medical or psychiatric problems or were unable to participate in all study procedures or provide informed consent. Written informed consent was obtained as approved by the NIH institutional review board after a complete description of the study to subjects was provided. Subjects were randomized to condition (aprepitant or placebo) upon enrollment in the study, which used a double-blind parallel group design with an intended allocation ratio of 1:1. An aprepitant dose of 125mg daily was chosen because prior PET studies have shown that doses above 100mg daily are required to achieve central receptor occupancy (RO) in excess of 90% (Bergstrom et al. 2004), commonly thought to be needed for adequate target engagement in CNS disorders (Frank and Hargreaves 2003). Higher doses, 300mg daily, were initially used in depression studies (Kramer et al. 1998), and were found to be safe and well tolerated, but subsequent studies used lower doses because of cost, and also because of a dose-dependent potential for pharmacokinetic interactions with contraceptives. Aprepitant was given for three weeks (21 days), following a one week placebo lead-in period. The total length of stay at NIH was approximately six weeks; one week for detoxification and eligibility determination, 32 days to participate in the study, and several days following study completion to prepare for discharge.
Subjects remained hospitalized throughout the study, and participated in standard-of-care behavioral alcoholism treatment throughout their stay. Upon inclusion, they were evaluated for alcoholism severity using the Alcohol Dependence Scale [ADS; (Skinner, 1984)], for alcohol consumption in the past 90 days using the Timeline Follow-Back [TLFB; (Sobell et al., 1986)], for personality traits using the NEO Personality Inventory Revised [NEO; (Costa, 2002)], and for early life adversity using the Childhood Trauma Questionnaire [CTQ; (Bernstein et al., 1994)]. PTSD symptoms were assessed upon entry to and completion of the study using the Clinician-Administered PTSD Scale [CAPS; (Weathers et al., 2001)], as well as weekly using the PTSD Symptom Severity Interview [PSSI; (Foa et al., 1993)].
A challenge procedure that combined the Trier Social Stress Test (Kirschbaum et al., 1993) and manipulation (handling and smelling, but not consuming) of each subject’s preselected alcoholic beverage as a cue (Stasiewicz et al., 1997), hereafter referred to as Trier/CR, was carried out as previously described (George et al., 2008; Kwako et al., 2014) around day 20 of the study. Challenge sessions using personalized auditory guided imagery scripts, each approximately five minutes in duration and presenting stress-, alcohol-associated or neutral stimuli as described (Sinha et al., 2011b) occurred on days 25 – 27. Both challenge procedures (i.e., Trier/CR and scripts) began at 3pm to minimize differences in diurnal cortisol output. During the challenge sessions, craving for alcohol was rated using the Alcohol Urge Questionnaire [AUQ; (Bohn et al., 1995)]. The Subjective Units of Distress Scale (SUDS), a visual analog scale (1–100), and the Spielberger State Anxiety Inventory [STAI-S; (Spielberger, 1970)] were used to assess self-reported distress and anxiety, respectively. ACTH and cortisol were used as endocrine stress markers. A graphical representation of the data collection time points and challenge procedures for the Trier/CR and scripts appears in Supplementary Figure 1.
We collected blood for cortisol in a 3cc serum separator tube (Vacuette Z serum separator clot activator #454067) at room temperature, and blood for ACTH in a 3cc EDTA tube (K2 EDTA 5.4mg #367856). The EDTA tubes were pre-chilled on wet ice and immediately returned to wet ice after collection. After procedures were completed, we sent samples to the NIH Department of Laboratory Medicine (CLIA ID Number 21D0665373) for immediate assay. Tests for serum cortisol were run using the IMMULITE 2000 Systems Analyzers with PIL2K/CO-20 kit, a solid-phase, competitive chemiluminescent enzyme immunoassay. The assay sensitivity was 0.20 μg/dL, with a published intraassay coefficient of variation (CV) average of 6.0% and interassay CV of 7.8%.mm. Tests for ACTH in EDTA plasma were run using the IMMULITE 2000 Systems Analyzers with PIL2KAC-15 kit, a solid-phase, two-site sequential chemiluminescent immunoassay. The assay sensitivity was 5 pg/mL, with a published intraassay CV average of 7.7% and interassay CV of 8.5%.
Finally, following the last challenge session and at least one day of recovery, subjects underwent an fMRI study. For a detailed description of fMRI data analysis see e.g. (George et al., 2008). Briefly, imaging was performed using a 3T General Electric MRI scanner with a 16-channel head coil. Imaging paradigms included the presentation of 55 high arousal negative and 55 high arousal positive pictures from the International Affective Picture System [IAPS; (Lang et al., 1999)]. Scrambled images were used as the control condition and were displayed during the inter-stimulus interval (ISI), which ranged between 0–15 seconds. The scrambled images were derived from the IAPS images and preserved overall brightness and color but did not contain recognizable features. Images were presented in a random order in one run lasting 9 minutes and 30 seconds. A second paradigm included pictures of alcoholic and neutral beverages pictures (e.g., milk, orange juice) presented in random order in a run lasting 9 minutes and 30 seconds. Each stimulus presentation lasted 800 milliseconds. Finally, 45 neutral and 45 fearful faces (Matsumoto and Ekman, 1988), as well as a non-emotional control cross-hair condition that served as the inter-stimulus interval, were presented in an event-related design that lasted 8 minutes and 30 seconds. Stimuli were presented for two seconds each, and the inter-stimulus interval ranged from 0 to 8 seconds. The three paradigms were presented in randomized and counter-balanced order across subjects.
fMRI data were analyzed using Analysis of Functional Neural Images (AFNI) software (Cox, 1996). Statistical maps were generated for each individual separately by linear contrasts between the regressors of interest (negative and positive IAPS images; alcoholic and not alcoholic beverages; neutral and fearful faces). Preprocessed time series data for each individual were then analyzed by multiple regression, which allowed covariation of variables related to head motion and scanning run. We also calculated a statistical map of the activation within each group (aprepitant and placebo) for each stimulus condition. Each condition was compared to the baseline scrambled image. We then performed voxel-wise t-tests of the event-related β-coefficients calculated from the general linear model to test for differences between the aprepitant group and the placebo group for each condition. A whole brain family-wise error rate correction using a Monte Carlo simulation was used to rule out false positives, yielding a corrected type I error rate < 0.05.
T-statistics from the group maps were subsequently characterized by an assessment of actual BOLD signal changes in volumes of interest (VOIs). We analyzed VOIs in the NAcc, anterior and posterior insula, amygdala, and medial frontal gyrus, based on prior literature implicating these regions as active during presentation of alcohol related cues and emotional stimuli in alcoholics and subjects with PTSD.
Behavioral data were analyzed using PROC MIXED for mixed-effect modeling in SAS version 9.3 (SAS Institute, Cary, NC), with treatment (aprepitant/placebo) as the fixed, between-subjects factor. Repeated within-subjects factors included time point (Trier/CR outcome measures), script context (neutral, alcohol cue, or stress/trauma) and time point (Scripts outcome measures), or day (weekly and biweekly measures). The level of statistical significance was set at p < .05 for all tests, and all post hoc comparisons were conducted using Tukey’s Honestly Significant Difference (HSD) test. Potential covariates were evaluated for inclusion on a model-by-model basis such that covariates that significantly predicted the outcome measure were retained in the model. Covariates that were evaluated included gender, race, age, alcohol dependence severity (ADS) score, number of heavy drinking days from the TLFB, total score from the CTQ, neuroticism score from the NEO, and the total score from the ASI. Model-specific covariates are noted in the relevant figure legends.
RESULTS
Participant Characteristics
Demographic data and other baseline characteristics appear in Table 1. In total, the study enrolled 60 participants, 53 of whom we have complete data on (as reported in the current study). Five individuals did not complete the study, including three who withdrew against medical advice for personal reasons, one who left due to increased stress while participating in the study, and a fifth who was discontinued after an initial positive screen for HIV (an exclusionary criterion for participation). Participants, including 24 women and 29 men, averaged about 40 years of age, consumed on average about 15 drinks per day in the 90 days preceding admission, and had alcoholism in the severe range according to the ADS. T-tests found no significant group differences in demographic, alcohol-related, or psychosocial characteristics. The most commonly reported side effects included headaches, nausea, fatigue, diarrhea, and lack of appetite; a more complete description of reported side effects appears in Supplementary Table 1. There were no group differences in the frequencies of reported side effects.
Table 1. Demographic data.
Demographic data, including age, gender, race, years of education, smoking status, and recent alcohol use data and psychological characteristics for all participants.
| Aprepitant (n = 26) | Placebo (n = 27) | Total (n = 531) | |
|---|---|---|---|
| Demographics | |||
| Age | 41.8 (8.3) | 39.8 (7.6) | 40.8 (7.9) |
| Female | 11 (42.3%) | 13 (48.1%) | 24 (45.3%) |
| Caucasian2 | 12 (46.2%) | 11 (40.7%) | 23 (43.4%) |
| Education (years) | 13.3 (2.6) | 13.3 (2.8) | 13.2 (2.6) |
| Smoker | 20 (76.9%) | 23 (85.2%) | 43 (81.1%) |
|
| |||
| Alcohol Use (past 90 days) | |||
|
| |||
| Average Drinks/day | 16.1 (9.5) | 14.7 (7.4) | 15.4 (8.4) |
| Heavy Drinking Days | 67.2 (23.9) | 63.7 (23.3) | 65.4 (23.4) |
| ADS Score | 22.8 (6.9) | 20.8 (8.6) | 21.8 (7.8) |
|
| |||
| Psychological Characteristics | |||
|
| |||
| CTQ total score | 53.0 (19.9) | 53.5 (17.9) | 53.3 (18.7) |
| Neuroticism score | 63.8 (9.9) | 60.3 (11.4) | 62.1 (10.7) |
| PSSI total severity | 34.7 (5.7) | 36.5 (9.3) | 35.5 (7.6) |
| CAPS total severity | 86.2 (16.3) | 85.8 (19.3) | 86.0 (17.7) |
| ASI total score | 2.7 (0.8) | 2.6 (0.8) | 2.7 (0.8) |
52 of the 53 subjects completed the Scripts and Trier/CR challenges; the remaining subject, while not completing the full Scripts procedure, was included in the analysis of CAPS data.
The majority of the remaining subjects were Black/African American
PTSD Symptoms
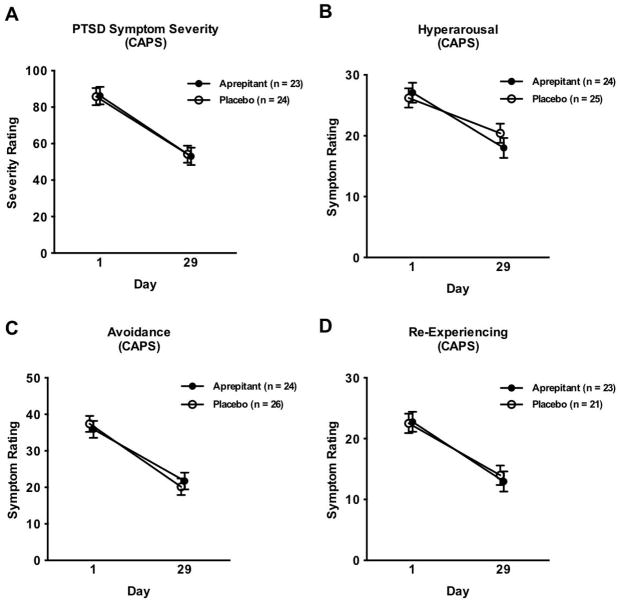
Linear mixed models found that PTSD symptom severity as measured by the CAPS declined from Day 1 to Day 29 of the study (F[1,43] = 67.07, p = 0.0001; Figure 1A); the same decline was seen for the symptom subtypes of hyperarousal (F[1,45] = 38.70; p = 0.0001; Figure 1B), avoidance (F[1,44] = 64.31; p = 0.0001; Figure 1C) and re-experiencing (F[1,45] = 30.61; p = 0.0001; Figure 1D). There was, however, no significant effect of treatment on overall PTSD symptom severity, or on any of the three symptom domains. Similar results were seen over time for total symptom severity measured using the PSSI (F[4, 155] = 21.06; p = 0.0001), and hyperarousal (F[4, 151] = 5.82; p = 0.0002), avoidance (F[4, 152] = 11.05; p = 0.0001), and re-experiencing symptoms (F[4, 164] = 13.22, p = 0.0001) measured with this assessment.
Figure 1. PTSD symptom severity measured by the CAPS.
A) Overall severity score. Covariates in the model included gender and total score from the ASI. The sample size for this model, as well as for the next three models, was reduced due to missing data for ASI total score. B) Hyperarousal. Covariates in the model included gender and total score from the ASI. C) Avoidance. Covariates in the model included gender and total score from the ASI. D) Re-experiencing. Covariates in the model included gender and total score from the ASI.
Craving
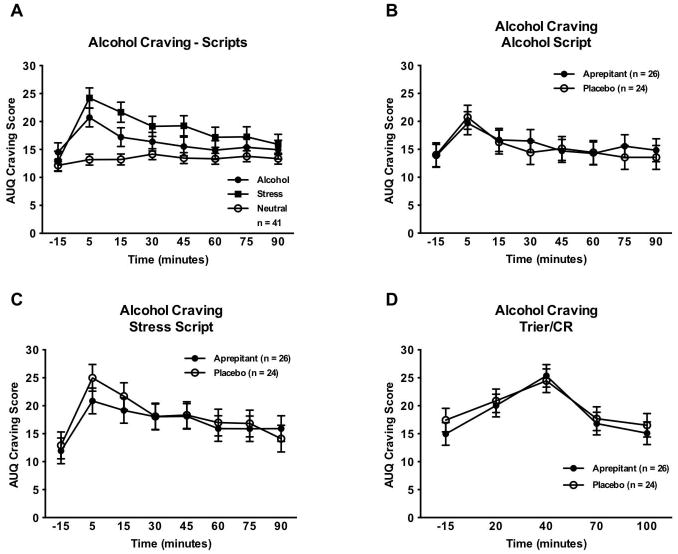
Linear mixed models indicated a significant main effect of script type on craving (F[2,80] = 5.29, p = 0.007); as expected, the stress and alcohol scripts both induced more craving for alcohol than did the neutral script (Figure 2A). There were, however, no significant effects of treatment on craving for alcohol as induced by the alcohol script (F[1,46] = 0.04, p = 0.85; Figure 2B) or stress script (F[1,45] = 0.13, p = 0.72; Figure 2C). Similarly, there was no effect of treatment on craving in response the Trier/CR (F[1,43] = 0.20, p = 0.66; Figure 2D).
Figure 2. Alcohol craving response to challenge procedures.
A) Effect of script type on alcohol craving. Covariates in the model included gender, race, ADS score, PTSD severity score at baseline (Day 1), and neuroticism. The sample size for this analysis was reduced due to missing data for baseline PTSD severity score for several of the subjects, and missing data for neuroticism for two of the placebo subjects. B) Effect of aprepitant treatment on craving response to the alcohol cue script. Covariates in the model included gender and neuroticism. The sample size for this analysis, as well as the analyses for the stress script and the Trier/CR, was reduced due to missing data for neuroticism for two of the placebo subjects. C) Effect of aprepitant treatment on craving response to the stress script. Covariates in the model included gender and neuroticism. D) Effect of aprepitant treatment on craving response to the Trier/CR. Covariates in the model included gender, race, ADS score, number of heavy drinking days, and neuroticism.
Stress and Anxiety
Linear mixed models also found a significant main effect of script type on subjective stress responses (F[2,98] = 7.01; p = 0.001); as expected the stress script induced higher distress than did either the neutral or alcohol script, which did not differ from each other. There were, however, no significant effects of treatment on the subjective stress response to either the alcohol script (F[1,46] = 0.10; p = 0.76); or the stress script (F[1,44] = 0.06; p = 0.80). Linear mixed models showed a marginally significant main effect of treatment on subjective distress induced by the Trier/CR; patients taking aprepitant reported slightly higher distress than those taking placebo (F[1,44] = 4.02; p = 0.052). There was also a significant effect of script type on anxiety (F[2,98] = 8.72; p = 0.001), as found by linear mixed models; as expected, the stress script induced more anxiety than did either the cue or neutral script, which did not differ from each other. There were, however, no significant effects of treatment on anxiety as induced by the cue script (F[1,49] = 0.13; p = 0.72); or stress script (F[1,46] = 0.22; p = 0.64). There was a trend toward a significant treatment effect on anxiety as induced by the Trier/CR (F[4,192] = 9.79; p = 0.09), such that the aprepitant group reported higher anxiety than the placebo group.
Endocrine Response
Linear mixed models found no significant main effects of script type (F[2,82] = 1.35; p = 0.27), or time (F[9,369] = 0.99; p = 0.45), nor any script type by time interaction (F[18,674] = 0.94; p = 0.53), on cortisol levels, indicating that there was no specific, dynamic endocrine response to any of the scripts (Supplementary Figure 2A). There was no significant effect of treatment on the cortisol response to the alcohol script (F[1,37] = 0.69; p = 0.41; Supplementary Figure 2B); there was, however, a significant main effect of treatment on cortisol levels during the stress script (F[1,37] = 5.18, p = 0.03; Supplementary Figure 2C). Specifically, throughout the stress script session participants receiving aprepitant had somewhat reduced cortisol levels compared to participants receiving placebo. There was no significant effect of treatment on cortisol during the Trier/CR (F[1,40] = 0.03; p = 0.86; Supplementary Figure 2D). Linear mixed models found no effects of script type (F[2,82] = 0.09; p = 0.92), or time (F[9,369] = 0.70; p = 0.71), nor any script type by time interaction (F[18,680] = 1.13; p = 0.32), on the ACTH response to the scripts (Supplementary Figure 3A). There was no significant effect of treatment on the ACTH response to the alcohol script (F[1,26] = 0.06; p = 0.81; Supplementary Figure 3B); or the stress script (F[1,41] = 0.96; p = 0.33; Supplementary Figure 3C), or on the Trier/CR (F[1,44] = 0.65; p = 0.43; Supplementary Figure 3D).
fMRI
General linear models found a significant main treatment effect on an area including both right and left vmPFC BOLD activation in response to negative IAPS pictures (negative – neutral). The aprepitant group showed significantly higher bilateral activation than the placebo group (whole brain corrected p = 0.01; n = 40 participants; cluster volume: 8×140/1000 ml; cluster size: 5; Figure 3). There were no significant effects of treatment on neural activation in response to fearful vs. neutral faces, or alcoholic vs. non-alcoholic beverages.
Figure 3. mPFC activation in response to negative IAPS images.
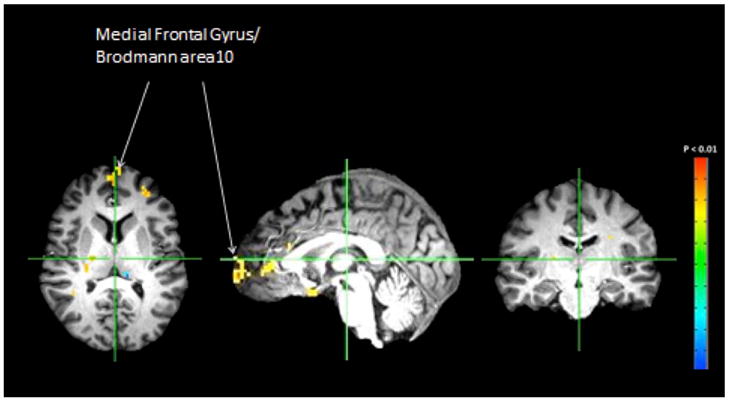
Relevant areas of activation are labeled.
DISCUSSION
NK1 antagonism has been reported to attenuate both stress responses and alcohol seeking in humans and experimental animals (George et al., 2008; Schank et al., 2011; Schank et al., 2012). We therefore evaluated the orally available, brain penetrant NK1 antagonist aprepitant in inpatients with co-morbid PTSD and alcoholism. The most salient finding was that aprepitant treatment resulted in a robust fMRI BOLD activation of an area including vmPFC when subjects were exposed to aversive visual stimuli. This finding was not accompanied by reduced PTSD symptom severity or attenuated stress-induced alcohol craving.
An impaired activity of the mPFC in response to aversive stimuli is one of the most consistent features of PTSD, and has been observed utilizing imaging modalities that include PET, SPECT, and fMRI [reviewed in (Liberzon and Sripada, 2008)]. Activity of the mPFC in PTSD has been reported to correlate inversely with that of the amygdala [see (Shin et al., 2005)], and also, in some studies, with clinical PTSD symptom severity, as measured by CAPS ratings (Shin et al., 2004). We did not evaluate PTSD or other behavioral responses to the fMRI data presented in the present study, so we are unable to link our fMRI data with such responses. The vmPFC areas that were most consistently activated during viewing of aversive stimuli in our study project to subregions of the amygdala, and can exert a top-down regulation of its activity. The activity of these vmPFC – amygdala projections may be important in the ability to differentiate between safe and unsafe conditions (Likhtik et al., 2014), as suggested by recent preclinical data, and is critical for extinguishing conditioned responses to stimuli that were previously associated with stress-induced alcohol craving and alcohol relapse (Seo et al., 2013), or a threat that is subsequently experienced in the absence of aversive outcomes [reviewed in (Quirk and Mueller, 2008)]. NK1 receptors modulate unconditioned fear and stress responses at several levels of the neuraxis, including the dorsal raphe, amygdala, and PFC [reviewed in (Schank et al., 2012)], but much less is known about their potential role in extinction of fear memories. Our imaging findings suggest the intriguing possibility that in PTSD, NK1 antagonism may be useful to facilitate behavioral exposure treatment aimed at extinguishing maladaptive stress responses, rather than to directly suppress responses to aversive stimuli. This possibility is supported by the literature cited above, although Milad and colleagues (2007) found a vital role of both the vmPFC and hippocampus in the extinction recall. We did not find enhanced hippocampal activation in the aprepitant group, however, the supposition that NK1 antagonism may enhance fear extinction, through the vmPFC-amygdala pathways, warrants further exploration.
Although speculative at this stage, this interpretation is consistent with our observations that, in the absence of PTSD-specific behavioral treatment, NK1 antagonism did not influence clinical or behavioral outcomes in the PTSD or alcohol domains. The lack of medication effect on the CAPS and its subscales and the PSSI and its subscales directly establishes a lack of efficacy to improve PTSD symptoms, at least within the timeframe examined. Although treatment duration was limited to four weeks, the low variance and near-identical outcomes between the aprepitant and placebo groups make it unlikely that an effect would emerge over longer treatment duration or with a larger sample. This conclusion is further supported by the observation that the study design had sufficient power to detect differential improvement between men and women.
In the alcoholism domain, we assessed alcohol craving as a surrogate marker of potential clinical efficacy, because it has previously been established that craving in response to the stress-associated stimuli used in our study is predictive of relapse during subsequent outpatient follow-up (Sinha et al., 2011b; Sinha et al., 2011a). As expected, both stress and alcohol scripts induced robust craving responses, with low variance. The utility of the guided imagery methodology was further illustrated by the observation that the stress scripts induced robust craving, which was accompanied by anxiety and distress responses. In contrast, the alcohol scripts induced equally robust craving responses, but did so in the absence of concomitant anxiety or distress. This dissociation parallels the convergent but distinct pathways to drug seeking and relapse in response to stress or drug cues, respectively, extensively outlined by preclinical research (Bossert et al., 2013). Thus, despite seemingly adequate measurement properties of our surrogate marker, we did not detect any signal for a treatment effect.
Our negative data on stress-induced alcohol craving in the present sample are in apparent contrast to a series of animal studies and one human trial suggesting a utility of NK1 antagonism to reduce stress-induced alcohol seeking and stress responses (George et al., 2008; Schank et al., 2011; Schank et al., 2012). Of note, however, the NK1 receptor antagonist L822429 had no effect on incubation of fear in rats (Schank et al., 2011), a potential animal model of delayed-onset PTSD (Pickens et al., 2009).
At least two possibilities must be considered in interpreting these seemingly divergent data. The first is whether our aprepitant dose produced a level of central NK1 RO sufficient to achieve behavioral effects. Our observation of a robust aprepitant effect on an fMRI-based outcome is likely to serve as a biomarker of target engagement, but might be more sensitive to NK1 blockade than behavioral outcomes. It is generally held that approximately 90% central RO is sufficient for efficacy with most CNS-active drugs, and PET displacement studies indicate that this level is reached in most patients with the 125 mg/day aprepitant dose and treatment duration used in our study (Frank and Hargreaves, 2003). This assumption is, however, purely empirical and accumulating data suggest that it may not hold up with regard to the SP/NK1 system. For example, an early study showed antidepressant efficacy of aprepitant at a daily dose of 300 mg (Kramer et al., 1998). Because of pharmacokinetic interactions at this high dose, and also because of the high cost of aprepitant synthesis, a follow-up study was carried out using a lower, 125 mg/day dose, but resulted in negative findings (Keller et al., 2006). More recently, positive results were obtained when the NK1 antagonist casopitant was used at 80 mg/day, a dose that results in near-complete central RO (Ratti et al., 2011; Zamuner et al., 2012). Taken together, these data suggest that seemingly inconsistent results in studies of NK1 antagonists for psychiatric indications may in part stem from a failure in some of the studies to achieve sufficient central RO. Similarly, the findings from the present study are somewhat limited in that we only tested one dosage of aprepitant, so are unable to assess its effects at different dosages.
Second, it is possible that the pathophysiology of patients with co-morbid PTSD and alcoholism differs from that of patients with alcoholism only, such that stress-induced cravings are induced through different mechanisms in these two populations. This explanation may be related to the unique pathology of HPA-axis function in PTSD. Cortisol responses to stressful stimuli have been found to vary among individuals with PTSD depending on the details of the challenge procedure (e.g, behavioral vs. pharmacological, timing, presence of co-morbidities) and features of the trauma exposure (de Kloet et al., 2006). The limited data comparing individuals with comorbid PTSD and AD with those with either disorder have not found differences in stress response to various challenge procedures between patient groups (Brady et al., 2006; McRae et al., 2006). Given the animal data showing that glucocorticoid signaling contributes to alcohol seeking, e.g., (Vendruscolo et al., 2012), it is possible that changes in glucocorticoid response among those with co-morbid PTSD and alcoholism may offer a mechanistic explanation for the lack of efficacy of NK1 antagonism to suppress stress-induced craving in the present sample as compared to our prior findings (George et al., 2008).
To conclude, in patients with co-morbid PTSD and alcoholism, we discovered an unexpected effect of NK1 antagonism to activate the vmPFC during exposure to aversive visual stimuli. Although there was no significant drug effect on the primary outcomes, our findings suggest that the circuitry subserving stress-induced alcohol craving in this population differs from that in uncomplicated alcoholism. The findings further suggest that NK1 antagonist therapy may have a potential to augment established behavioral exposure-extinction treatment, rather than being active as a mono-therapy.
Supplementary Material
Supplementary Figure 1 Timeline of challenge procedures
Graphical representation of Trier/CR and Script procedures. The numbers represent minutes, i.e., in relation to the initiation of the procedure. S = scales, B = blood samples, V = vital signs.
Supplementary Figure 2 Cortisol response to challenge procedures
Due to missing data for cortisol levels, the sample sizes are reduced for these analyses. A) Effect of script type on cortisol levels. Covariates in the model included gender, ADS score, and total score from the ASI. B) Effect of aprepitant treatment on cortisol levels in response to the alcohol cue script. Covariates in the model included gender, ADS score, and total score from the ASI. C) Effect of aprepitant treatment on cortisol levels in response to the stress script. Covariates in the model included gender, ADS score, and total score from the ASI. D) Effect of aprepitant treatment on cortisol levels in response to the Trier/CR. Covariates in the model included gender, race, ADS score, and neuroticism.
Supplementary Figure 3 ACTH response to challenge procedures
Due to missing data for ACTH levels, the sample sizes are reduced for these analyses. A) Effect of script type on ACTH levels. Covariates in the model included gender, number of heavy drink days, total score from the CTQ, and total score from the ASI. B) Effect of aprepitant treatment on ACTH levels in response to the alcohol cue script. Covariates in the model included gender, age, overall PSSI severity score, CTQ total score, and total score from the ASI. C) Effect of aprepitant treatment on ACTH levels in response to the stress script. Covariates in the model included gender. D) Effect of aprepitant treatment on ACTH levels in response to the Trier/CR. Covariates in the model included gender and number of heavy drink days.
Supplementary Table 1 Reported side effects
This table includes side effects reported by participants, organized by group. Data are reported for all subjects for 10 side effects, and eight additional side effects are reported for a subset of participants. These side effects were added to those collected part-way through the study, which accounts for the reduced sample size.
Acknowledgments
This work was carried out under a Clinical Trial Agreement (CTA) between the US Government and Merck & Company, Incorporated. Merck had no role in study design, collection, analysis or interpretation of the data, or drafting of the manuscript. Drs. Kwako, George, Schwandt, Spagnolo, Momenan, Hommer, Shaham, and Heilig, as well as Ms. Diamond, declare no competing financial interests. Dr. Sinha is a member of the Embera Neurotherapeutics Scientific Advisory Board. This research was supported by the Division of Intramural Clinical and Biological Research, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health. We thank the following individuals and departments: Lauren Adams, Joanna Sells, Jessica Berman, Eric Markey, Victoria Brown, Keva Garg, and Byung Joon Park; Debra Hill, Cheryl Jones, and Monte Phillips; and James Paterson, Judie Jones, Mary Ley, Jacqueline Goodson, and the 1SE Nursing Staff of the NIH Clinical Center.
References
- Acheson DT, Gresack JE, Risbrough VB. Hippocampal dysfunction effects on context memory: possible etiology for posttraumatic stress disorder. Neuropharmacology. 2012;62:674–685. doi: 10.1016/j.neuropharm.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcoholism: Clinical and Experimental Research. 2005;29:1351–1355. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom M, Hargreaves RJ, Burns HD, et al. Human positron emission tomography studies of brain neurokinin 1 receptor occupancy by aprepitant. Biol psychiatry. 2004;55:1007–1012. doi: 10.1016/j.biopsych.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiat. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcoholism: Clinical and Experimental Research. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Waldrop AE, McRae AL, Back SE, Saladin ME, Upadhyaya HP, Anton RF, Randall PK. The impact of alcohol dependence and posttraumatic stress disorder on cold pressor task response. J Stud Alcohol. 2006;67:700–706. doi: 10.15288/jsa.2006.67.700. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Stasiewicz PR, Hughes PM, Brimo ML. Trauma-focused imaginal exposure for individuals with comorbid posttraumatic stress disorder and alcohol dependence: revealing mechanisms of alcohol craving in a cue reactivity paradigm. Psychol Addict Behav. 2006;20:425–435. doi: 10.1037/0893-164X.20.4.425. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Schumacher JA, Stasiewicz PR, Henslee AM, Baillie LE, Landy N. Craving and physiological reactivity to trauma and alcohol cues in posttraumatic stress disorder and alcohol dependence. Exp Clin Psychopharmacol. 2010;18:340–349. doi: 10.1037/a0019790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol. 1997;106:243–250. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- Costa PTM, RR . NEO Personality Inventory-Revised (NEO PI-R) Washington, DC: APA; 2002. [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HG. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. Journal of psychiatric research. 2006;40:550–567. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Patient Edition. New York: Biometric Research; 1995. [Google Scholar]
- Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and Validity of a Brief Instrument for Assessing Posttraumatic-Stress-Disorder. J Trauma Stress. 1993;6:459–473. [Google Scholar]
- Fox HC, Bergquist KL, HK-I, Sinha R. Stress-induced and alcohol cue-induced craving in recently-abstinent alcohol dependent individuals. Alcoholism: Clinical and Experimental Research. 2007;31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Frank R, Hargreaves R. Clinical biomarkers in drug discovery and development. Nature reviews Drug discovery. 2003;2:566–580. doi: 10.1038/nrd1130. [DOI] [PubMed] [Google Scholar]
- George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, Peng X, Kielbasa W, Rawlings R, Brandt JE, Gehlert DR, Tauscher JT, Hunt SP, Hommer D, Heilig M. Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science. 2008;319:1536–1539. doi: 10.1126/science.1153813. [DOI] [PubMed] [Google Scholar]
- Geracioti TD, Jr, Carpenter LL, Owens MJ, Baker DG, Ekhator NN, Horn PS, Strawn JR, Sanacora G, Kinkead B, Price LH, Nemeroff CB. Elevated cerebrospinal fluid substance p concentrations in posttraumatic stress disorder and major depression. The American journal of psychiatry. 2006;163:637–643. doi: 10.1176/ajp.2006.163.4.637. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Phifer J, Bradley B, Ressler KJ. Risk and resilience: genetic and environmental influences on development of the stress response. Depress Anxiety. 2009;26:984–992. doi: 10.1002/da.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley AE, Crane NA, Spadoni AD, Quello SB, Goodell V, Mason BJ. Craving in response to stress induction in a human laboratory paradigm predicts treatment outcome in alcohol-dependent individuals. Psychopharmacology (Berl) 2011;218:121–129. doi: 10.1007/s00213-011-2355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Committee on Treatment of Posttraumatic Stress Disorder. Treatment of posttraumatic stress disorder: an assessment of the evidence. Washington, D.C: National Academies Press; 2008. [Google Scholar]
- Keller M, Montgomery S, Ball W, Morrison M, Snavely D, Liu G, Hargreaves R, Hietala J, Lines C, Beebe K, Reines S. Lack of efficacy of the substance p (neurokinin1 receptor) antagonist aprepitant in the treatment of major depressive disorder. Biological psychiatry. 2006;59:216–223. doi: 10.1016/j.biopsych.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of general psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kramer MS, et al. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science. 1998;281:1640–1645. doi: 10.1126/science.281.5383.1640. [DOI] [PubMed] [Google Scholar]
- Kwako LE, Schwandt ML, Sells JR, Ramchandani VA, Hommer DW, George DT, Sinha R, Heilig M. Methods for inducing alcohol craving in individuals with comorbid alcohol dependence and posttraumatic stress disorder: Behavioral and physiological outcomes. Addict biol. 2014 doi: 10.1111/adb.12150. (e-publication ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Technical manual and affective ratings. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 1999. [Google Scholar]
- Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Stujenske JM, Topiwala MA, Harris AZ, Gordon JA. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci. 2014;17:106–113. doi: 10.1038/nn.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcoholism: Clinical and Experimental Research. 2000;24:651–658. [PubMed] [Google Scholar]
- Matsumoto D, Ekman P. Japanese and Caucasian facial expressions of emotion and neutral faces (JACFEE and JACNeuF) Human Interaction Laboratory, University of California; San Francisco: 1988. p. 401. [Google Scholar]
- McRae AL, Saladin ME, Brady KT, Upadhyaya H, Back SE, Timmerman MA. Stress reactivity: biological and subjective responses to the cold pressor and Trier Social stressors. Hum Psychopharmacol. 2006;21:377–385. doi: 10.1002/hup.778. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Mills KL, Teeson M, Back SE, Brady KT, Baker AL, Hopwood S, Sannibale C, Barrett EL, Merz S, Rosenfeld J, Ewer PL. Integrated exposure-based therapy for co-occurring posttraumatic stress disorder and substance dependence: a randomized controlled trial. JAMA. 2012;308:690–699. doi: 10.1001/jama.2012.9071. [DOI] [PubMed] [Google Scholar]
- Monti PM, Binkoff JA, Abrams DB, Zwick WR, Nirenberg TD, Liepman MR. Reactivity of alcoholics and nonalcoholics to drinking cues. J Abnorm Psychol. 1987;96:122–126. doi: 10.1037//0021-843x.96.2.122. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Golden SA, Adams-Deutsch T, Nair SG, Shaham Y. Long-lasting incubation of conditioned fear in rats. Biol Psychiatry. 2009;65:881–886. doi: 10.1016/j.biopsych.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratti E, Bellew K, Bettica P, Bryson H, Zamuner S, Archer G, Squassante L, Bye A, Trist D, Krishnan KR, Fernandes S. Results from 2 randomized, double-blind, placebo-controlled studies of the novel NK1 receptor antagonist casopitant in patients with major depressive disorder. J Clin Psychopharmacol. 2011;31:727–733. doi: 10.1097/JCP.0b013e31823608ca. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Rubonis AV, Sirota AD, Niaura RS, Colby SM, Wunschel SM, Abrams DB. Cue reactivity as a predictor of drinking among male alcoholics. J Consult Clin Psychol. 1994;62:620–626. doi: 10.1037//0022-006x.62.3.620. [DOI] [PubMed] [Google Scholar]
- Santa Ana EJ, Saladin ME, Back SE, Waldrop AE, Spratt EG, McRae AL, LaRowe SD, Timmerman MA, Upadhyaya H, Brady KT. PTSD and the HPA axis: differences in response to the cold pressor task among individuals with child vs. adult trauma. Psychoneuroendocrinology. 2006;31:501–509. doi: 10.1016/j.psyneuen.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Schank JR, Ryabinin AE, Giardino WJ, Ciccocioppo R, Heilig M. Stress-related neuropeptides and addictive behaviors: beyond the usual suspects. Neuron. 2012;76:192–208. doi: 10.1016/j.neuron.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Pickens CL, Rowe KE, Cheng K, Thorsell A, Rice KC, Shaham Y, Heilig M. Stress-induced reinstatement of alcohol-seeking in rats is selectively suppressed by the neurokinin 1 (NK1) antagonist L822429. Psychopharmacology (Berl) 2011;218:111–119. doi: 10.1007/s00213-011-2201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, Sinha R. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA psychiatry. 2013;70:727–739. doi: 10.1001/jamapsychiatry.2013.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Shaham Y, Heilig M. Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology (Berl) 2011a;218:69–82. doi: 10.1007/s00213-011-2263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry. 2011b;68:942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA. Assessing Alcohol-Use by Patients in Treatment. Res Adv Alcohol Drug. 1984;8:183–207. [Google Scholar]
- Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E. The reliability of a timeline method for assessing normal drinker college students’ recent drinking history: utility for alcohol research. Addict Behav. 1986;11:149–161. doi: 10.1016/0306-4603(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. The State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- Stasiewicz PR, Gulliver SB, Bradizza CM, Rohsenow DJ, Torrisi R, Monti PM. Exposure to negative emotional cues and alcohol cue reactivity with alcoholics: a preliminary investigation. Behav Res Ther. 1997;35:1143–1149. [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety. 2001;13:132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Woodward SH, Kaloupek DG, Streeter CC, Martinez C, Schaer M, Eliez S. Decreased anterior cingulate volume in combat-related PTSD. Biological psychiatry. 2006;59:582–587. doi: 10.1016/j.biopsych.2005.07.033. [DOI] [PubMed] [Google Scholar]
- Zamuner S, Rabiner EA, Fernandes SA, Bani M, Gunn RN, Gomeni R, Ratti E, Cunningham VJ. A pharmacokinetic PET study of NK(1) receptor occupancy. European journal of nuclear medicine and molecular imaging. 2012;39:226–235. doi: 10.1007/s00259-011-1954-2. [DOI] [PubMed] [Google Scholar]
- Zhang W, Davidson JR. Post-traumatic stress disorder: an evaluation of existing pharmacotherapies and new strategies. Expert opinion on pharmacotherapy. 2007;8:1861–1870. doi: 10.1517/14656566.8.12.1861. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Timeline of challenge procedures
Graphical representation of Trier/CR and Script procedures. The numbers represent minutes, i.e., in relation to the initiation of the procedure. S = scales, B = blood samples, V = vital signs.
Supplementary Figure 2 Cortisol response to challenge procedures
Due to missing data for cortisol levels, the sample sizes are reduced for these analyses. A) Effect of script type on cortisol levels. Covariates in the model included gender, ADS score, and total score from the ASI. B) Effect of aprepitant treatment on cortisol levels in response to the alcohol cue script. Covariates in the model included gender, ADS score, and total score from the ASI. C) Effect of aprepitant treatment on cortisol levels in response to the stress script. Covariates in the model included gender, ADS score, and total score from the ASI. D) Effect of aprepitant treatment on cortisol levels in response to the Trier/CR. Covariates in the model included gender, race, ADS score, and neuroticism.
Supplementary Figure 3 ACTH response to challenge procedures
Due to missing data for ACTH levels, the sample sizes are reduced for these analyses. A) Effect of script type on ACTH levels. Covariates in the model included gender, number of heavy drink days, total score from the CTQ, and total score from the ASI. B) Effect of aprepitant treatment on ACTH levels in response to the alcohol cue script. Covariates in the model included gender, age, overall PSSI severity score, CTQ total score, and total score from the ASI. C) Effect of aprepitant treatment on ACTH levels in response to the stress script. Covariates in the model included gender. D) Effect of aprepitant treatment on ACTH levels in response to the Trier/CR. Covariates in the model included gender and number of heavy drink days.
Supplementary Table 1 Reported side effects
This table includes side effects reported by participants, organized by group. Data are reported for all subjects for 10 side effects, and eight additional side effects are reported for a subset of participants. These side effects were added to those collected part-way through the study, which accounts for the reduced sample size.