Abstract
Opioid receptors were shown to modulate a variety of cellular processes in the vertebrate central nervous system, including synaptic transmission. While the effects of opioid receptors on chemically mediated transmission have been extensively investigated, little is known of their actions on gap junction-mediated electrical synapses. Here we report that pharmacological activation of mu-opioid receptors led to a long-term enhancement of electrical (and glutamatergic) transmission at identifiable mixed synapses on the goldfish Mauthner cells. The effect also required activation of both dopamine D1/5 receptors and postsynaptic cAMP-dependent protein kinase A, suggesting that opioid-evoked actions are mediated indirectly via the release of dopamine from varicosities known to be located in the vicinity of the synaptic contacts. Moreover, inhibitory inputs situated in the immediate vicinity of these excitatory synapses on the lateral dendrite of the Mauthner cell were not affected by activation of mu-opioid receptors, indicating that their actions are restricted to electrical and glutamatergic transmissions co-existing at mixed contacts. Thus, as their chemical counterparts, electrical synapses can be a target for the modulatory actions of the opioid system. Because gap junctions at these mixed synapses are formed by fish homologs of the neuronal connexin 36, which is widespread in mammalian brain, it is likely that this regulatory property applies to electrical synapses elsewhere as well.
Keywords: gap junction, connexin 36, connexin 35, Mauthner cell, dopamine, auditory, teleost
the opioid system is formed by a family of endogenous peptides released by neurons with reported actions in virtually all physiological activities (Stevens 2009). From a general point of view, the opioid system seems to be involved in the modulation and adaptation of the organism to challenges by influencing both physiological and behavioral processes (Dreborg et al. 2008; Stevens 2009). Endogenous opioids such as enkephalins, dynorphins, and endorphins exert their actions through activation of mu, delta, and kappa types of receptors (Childers 1991; Williams et al. 2001). Because these receptors can also be activated exogenously by alkaloid opiates such as morphine and heroine (Williams et al. 2001) they became critical targets for the treatment of pain and contribute to mechanisms of drug addiction. Both the analgesic and addictive properties of morphine are abolished in mice lacking the mu-opioid receptor (MOR), suggesting that MORs mediate the therapeutic and adverse activities of this compound (Matthes et al. 1996). At the cellular level, opioid receptors are distributed at presynaptic and postsynaptic sites (Williams et al. 2001). By modulating Ca2+ channel conductances at presynaptic sites and K+ channel conductances at postsynaptic sites, the activation of these G protein-coupled receptors generally leads to a decrease of neurotransmitter release (Brundege and Williams 2002; Manzoni and Williams 1999; Williams et al. 2001) or to membrane potential hyperpolarization (Oleskevich et al. 1993; Williams et al. 2001), respectively.
In contrast to chemical transmission, the effect of opioid on electrical synapses remains largely unknown. Electrical synapses are a modality of synaptic transmission that is mediated by structures known as “gap junctions” (Goodenough and Paul 2009), clusters of intercellular channels that provide a conduit for the exchange of low molecular weight metabolites and offer a pathway of low resistance for the spread of electrical currents between neurons (Bennett and Zukin 2004; Connors and Long 2004; Pereda et al. 2013) and other excitable cell types (Veeraraghavan et al. 2014). While originally perceived as less prominent in mammals, electrical transmission was shown to be ubiquitous in both invertebrate and vertebrate nervous systems, including mammals (Connors and Long 2004; Pereda 2014; Shimizu and Stopfer 2013). Rather than static, this modality of interneuronal communication is highly dynamic, and the strength of electrical synapses was shown to be under the control of a variety of regulatory processes (Bloomfield and Völgyi 2009; O'Brien 2014; Pereda 2014; Pereda et al. 2013). Interestingly, a wealth of data indicates the widespread distribution of electrical synapses in structures of the vertebrate reward system (Vandecasteele et al. 2007), such as the ventral tegmental area (Allison et al. 2006), nucleus accumbens (O'Donnell and Grace 1993; Onn and Grace 2000), striatum (Vandecasteele et al. 2005, 2007; Venance et al. 2004), and prefrontal cortex (Onn and Grace 2000; Sun et al. 2012), at which MORs are known to be present and thought to play important functional roles (Williams et al. 2001).
To investigate the possible role of opioids in regulating electrical transmission we tested the effects of MOR activation on synaptic transmission at mixed, electrical and chemical, synapses on the goldfish Mauthner (M-) cells known as “large myelinated club endings” (club endings) (Bartelmez and Hoerr 1933; Furshpan 1964; Lin and Faber 1988; Robertson et al. 1963). The M-cells (a pair of unusually large reticulospinal neurons located in the medulla of fish) and its spinal network organize and mediate tail-flip escape responses that are critical for the survival of a fish (Faber and Pereda 2011). Club endings are anatomically and physiologically identifiable synaptic terminals that provide valuable auditory information to the M-cells for the initiation of escape responses and they have become a valuable model for the study of vertebrate electrical transmission (Pereda et al. 2004), as their gap junctions are formed by fish homologs of the neuronal connexin 36 (Cx36) (Pereda et al. 2003; Rash et al. 2013), which is widespread in the mammalian brain (Condorelli et al. 1998, 2000; Connors and Long 2004). From the evolutionary point of view, opioid receptors are duplicates that arise from a single ancestral gene (Stevens 2009). The complete vertebrate opioid system is already established at the first jawed vertebrates (Dreborg et al. 2008; Stevens 2009), and, among all the opioid receptors, the MOR shows evidence of rapid adaptive evolution (Stevens 2009) and was reported to be present in teleosts (Barrallo et al. 2000; Marron Fdez de Velasco et al. 2009). Here we report that activation of MORs led to a long-term enhancement of electrical (and glutamatergic) transmission at these synapses. The synaptic potentiation also required activation of dopamine D1/5Rs and involved the actions of postsynaptic cAMP-dependent protein kinase A (PKA), suggesting that opioid effects are mediated indirectly via release of dopamine from nearby dopaminergic varicosities. Thus, as chemical synapses, electrical synapses can be a target for the modulatory actions of the endogenous opioid system and exogenous opioids and contribute to mechanisms of drug addiction.
MATERIAL AND METHODS
Protocol approval.
Animals were used according to protocols approved by the Institutional Animal Care and Use Committees of the Albert Einstein College of Medicine and the Marine Biological Laboratory (in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals) with minimization of the numbers of animals used.
Electrophysiology.
In vivo intracellular recordings from M-cells were obtained in adult goldfish (Carassius auratus, 3–5 inches length) and carried out as described previously (Pereda et al. 1994). Voltage responses to electrical stimulation of the posterior VIIIth nerve or spinal cord were recorded from the lateral dendrite of the M-cell ∼350 μm from the M-cell axon's initial segment (Fig. 1A) via glass microelectrodes (4–7 MΩ) containing 5 M K-acetate (pH 7.2). To activate the auditory afferents, we placed a tungsten bipolar stimulating electrode on the posterior branch of the VIIIth nerve, where the saccular afferents that terminate as club endings run. The brief membrane time constant of the M-cell (∼400 μs) (Fukami et al. 1965) allows for both components of the mixed synaptic response to be unambiguously identified and reliably measured, as the brief duration of the electrical synaptic component does not significantly influence the peak of the delayed glutamatergic synaptic response (Fig. 1B). To obtain “100 Hz potentiation” of the mixed synaptic responses, a train of high-frequency stimulation of the posterior VIIIth nerve, consisting of 100 pulses at 100 Hz was applied five times every 4 s (Cachope et al. 2007). Synaptic responses were monitored every 4 s. For graphing and analysis, traces were averaged in sets of 10 or more. Changes in peak amplitude for each component of the synaptic responses were estimated by comparing averaged values obtained during the baseline recording with those obtained during the last 10 min of each experimental manipulation.
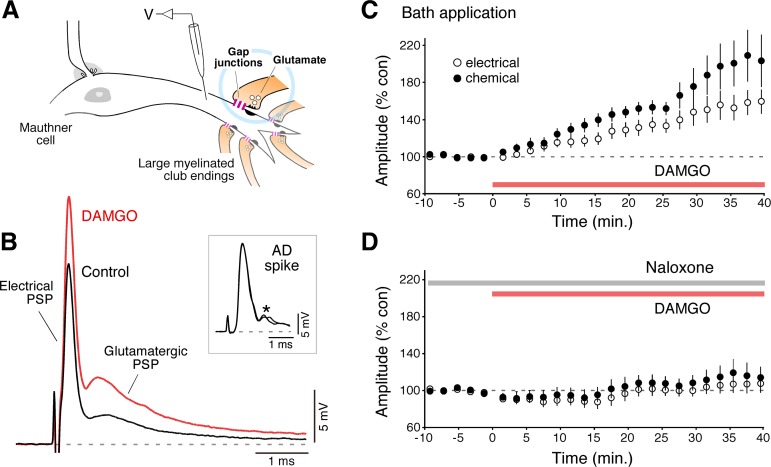
Fig. 1.
Bath application of DAMGO led to long-term potentiation of electrical and chemical transmission in club endings. A: club endings are single terminations of saccular afferents in the lateral dendrite of the Mauthner (M-) cell. Both gap junctions (gap junctions) and glutamatergic synapses (glutamate) coexist at these contacts. Intradendritic recordings were used to monitor the amplitude (V) of the mixed synaptic potential evoked by stimulation of the posterior branch of the VIIIth nerve and the antidromic (AD) spike evoked by stimulation of the M-cell axon in the spinal cord (see below). B: bath application of DAMGO led to a long-term potentiation of both components [Electrical postsynaptic potential (PSP) and Glutamatergic PSP] of the mixed synaptic response potential evoked by stimulation of the posterior branch of the VIIIth nerve. Inset: no significant changes were observed in the amplitude of the antidromic spike of the M-cell, and indicator of this cell's input resistance (see material and methods). Here and elsewhere traces represent the average of 10–15 individual responses. The AD spikes obtained before and after DAMGO application are illustrates superimposed. *Synaptic potentials evoked by spinal inputs to the M-cell during antidromic stimulation. C: averaged time course of the electrical (○) and chemical (●) components of the mixed synaptic potential following bath application of DAMGO (10 μM, red bar). D: pretreatment with Naloxone (10 μM, gray bar) blocked DAMGO-evoked potentiation.
Antidromic stimulation of the M-cell was obtained by placing a bipolar stimulating electrode in the spinal cord, in close proximity to the M-cell axon. The amplitude of the M-cell antidromic action potential was routinely monitored and taken as an indicator of the cell's input resistance. Because of the lack of active properties, the antidromic (AD) spike propagates passively from the initial segment along the somatodendritic membrane, and therefore its amplitude can change as a result of variations in membrane conductance, such as those produced by opening or closing of ion channels. Given the low input resistance of the M-cell, testing changes with a pulse requires large current injections that are difficult to obtain reliably with the recording electrode, as they tend to rectify with large current pulses, making it difficult to balance the bridge. The AD spike provides a quick and reliable way to test changes in the resistance of the M-cell, a procedure that has been previously validated (Furukawa and Furshpan 1963; Oda et al. 1995). The strength of the feedback inhibition was expressed as “fractional conductance” ratio, defined as (V/V′) − 1 (Oda et al. 1995) (Fig. 4). V and V′ were obtained by measuring amplitude changes in response to paired antidromic action spike stimulation (6 ms interval). The reduction in the amplitude of the second spike (V′) is proportional to the increase in membrane conductance (shunting) produced by the opening of ligand-gated Cl− channels triggered by the first, conditioning, AD spike (V).
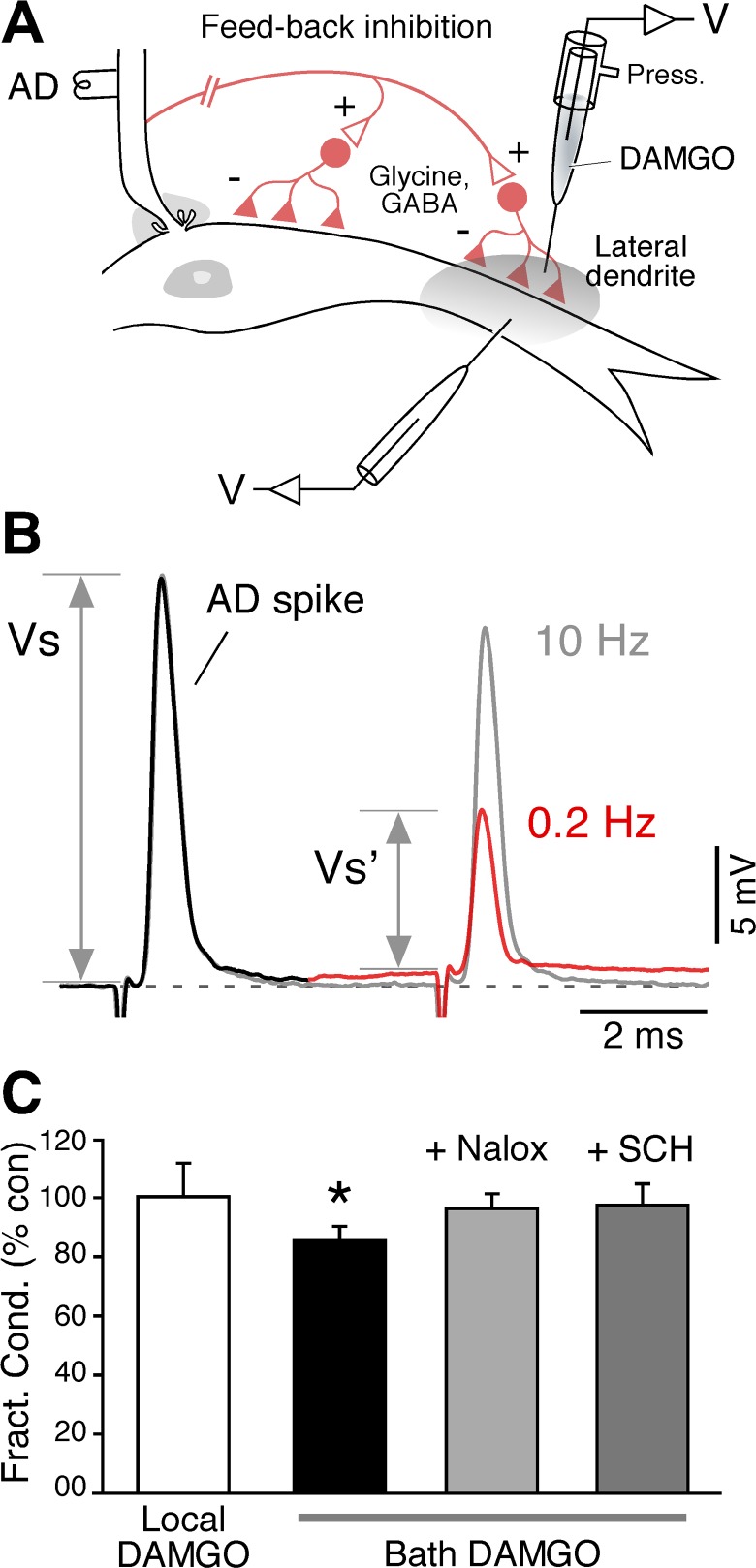
Fig. 4.
Inhibitory synaptic transmission in the vicinity of Club endings is not modified by local DAMGO application. A: experimental arrangement. The cartoon illustrates the inhibitory interneurons (Glycine, GABA) mediating feedback inhibition on the M-cell soma and lateral dendrite that are activated by antidromic stimulation (AD) of the M-cell axon. B: because the resting potential of the M-cell lies near the equilibrium potential for Cl−, no detectable hyperpolarization usually follows the antidromic stimulation of the M-cell axon. A 2nd AD spike (at a 6 ms interval) can be used to monitor the strength of the input, as this spike propagates passively through the somato-dendritic membrane, and therefore its amplitude is affected by changes in the cell's membrane conductance. Paired AD stimulation at 0.2 Hz evokes a dramatic reduction in the 2nd spike. The reduction in the 2nd spike is alleviated by stimulating at 10 Hz, which induces fatigue at M-cell output synapses, thus failing to activate the feedback inhibitory circuit, indicating that the reduction corresponded to an increase in membrane conductance produced by the opening of Cl− channels. The strength of the feedback inhibition was quantified by determining the “fractional conductance” (expressed as percentage of control), a ratio between the amplitudes of the AD spikes in the presence (Vs′) and absence (Vs) of feedback inhibition. C: summary of the effects. In contrast to local application, bath application of DAMGO modified the strength of feedback inhibition (fractional conductance is expressed as percentage of control; asterisk denotes significant change). The inclusion of Naloxone or SCH-23390 in the perfusion solution prevented this effect.
Drug application.
[d-Ala2, NMe-Phe4, Gly-ol5]-enkephalin (DAMGO, 10 μM; Tocris), Naloxone (10 μM, Tocris), and SCH-23390 (50 μM, Biomol) were bath-applied by superfusion of the brain after dilution in artificial cerebrospinal fluid (CSF). In the case of local application, a second recording microelectrode (12–16 MΩ) containing DAMGO was positioned extracellularly 300–400 μm from the axon hillock, 20–30 μm above the M-cell lateral dendrite. DAMGO (100 μM, Tocris) was dissolved in a vehicle solution consisting of 130 mM NaCl and 10 mM HEPES, pH 7.2, and pressure-ejected (Picospritzer II; Parker Instrumentation, Cleveland, OH) with five 20 psi pulses of 3 s duration each. Extracellular application of the vehicle solution was used in previous studies (Cachope et al. 2007; Pereda et al. 1992, 1994), and it does not alter the synaptic responses or the M-cell excitability. For intracellular application of the PKA inhibitor 5-24, this peptide (500 μM, Calbiochem) was dissolved in 0.5 M KCl and 10 mM HEPES, pH 7.2, and this solution was pressure injected in the M-cell lateral dendrite prior to bath application of DAMGO.
Statistical analysis.
Results are expressed as means ± SE. Paired Student's t-test was used to assess statistical significance of the data, unless otherwise stated (level of significance: 5%).
RESULTS
Club endings are single terminations of auditory afferents that originate in the sacculus, the auditory organ in fish. Consistent with the presence of gap junctions and specializations for chemical transmission at these terminals (Fig. 1A), the stimulation of the posterior branch of the VIIIth nerve (which contains the saccular afferents) evokes a mixed synaptic response [mixed excitatory postsynaptic potential (EPSP)] in the lateral dendrite of the M-cell composed of an early and brief depolarization representing the coupling potential of presynaptic action potentials (electrical component), which is followed by a delayed and longer-lasting glutamatergic response (Fig. 1B) and that represents the activation of a variable number of these terminals (chemical component). Given the brief time constant of the M-cell (∼400 μs), both components of the synaptic response can be unambiguously identified and reliably measured under various experimental conditions. To investigate the role of opioids in the M-cell system, we asked if activation of MORs could lead to changes in the amplitude of the mixed EPSP evoked by stimulation of the VIIIth nerve. We found that bath application of a CSF solution containing the MOR agonist DAMGO (10 μM) evoked a persistent enhancement of the electrical and chemical components of the mixed EPSP (Fig. 1, B and C) averaging 155.4 ± 14.1%, P < 0.006, and 199.5 ± 25.4%, P < 0.005, of control values, respectively (n = 8). Strikingly, the observed enhancement was not restricted to the glutamatergic transmission, known to be the target of the regulatory actions of opioids, but also included a parallel increase in electrical transmission. The observed enhancements cannot be ascribable to a change in dendritic conductance as the amplitude of the antidromic spike of the M-cell, an indicator of this cell's input resistance (see material and methods), was not significantly changed (Fig. 1B), averaging 107.4 ± 4.9% SE (P > 0.2) of control. This enhancement was prevented by pretreatment with the opioid antagonist Naloxone (10 μM), confirming that the effect of DAMGO was mediated by opioid receptor activation (Fig. 1D, n = 5), averaging 106.3 ± 8.7%, P > 0.5, and 115.3 ± 11.5%, P > 0.2, of control values for the electrical and chemical components, respectively (n = 5).
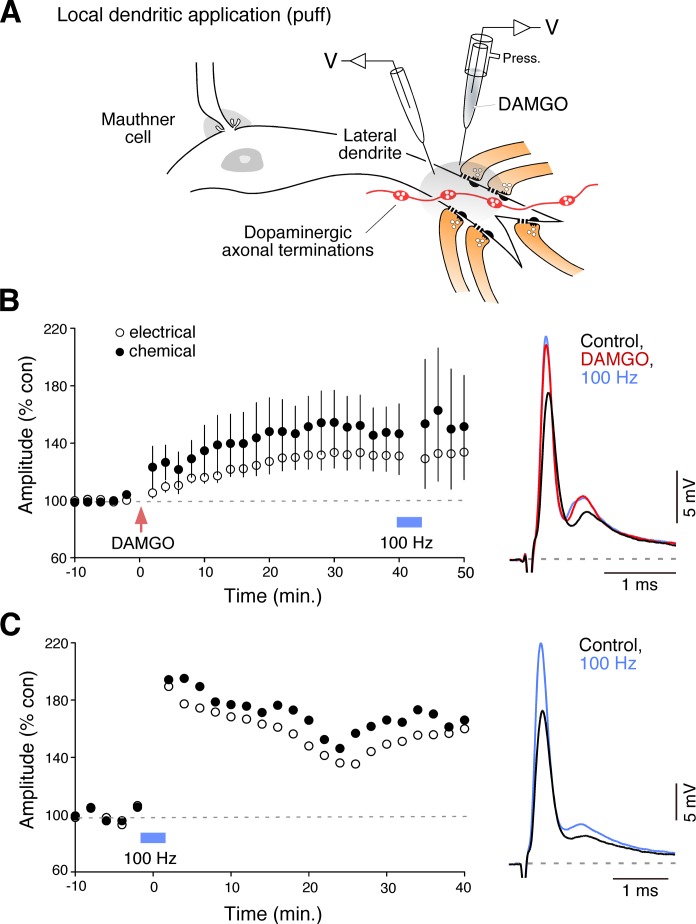
Because bath application of DAMGO also affect areas other than the club endings and thus effects can be mediated indirectly by actions on remotely located targets, we performed local applications of DAMGO (100 μM) on the lateral dendrite of the M-cell (Fig. 2A). Brief application of DAMGO with the help of a second recording electrode (puff) in the vicinity of the club endings triggered a long-term enhancement of the mixed EPSP (Fig. 2B). Following local DAMGO application, the electrical and chemical components averaged 131.8 ± 11.4%, P < 0.05 and 148.6 ± 20.1%, P < 0.05 of control values, respectively (n = 5). Because a brief application of DAMGO was sufficient to evoke a long-term effect on the mixed EPSP, the result suggests that MORs act as a trigger for long-term potentiation of these synapses. Consistent with this observation, the DAMGO-evoked potentiation was not reversed when Naloxone was added to the bath solution, indicating that activation of MORs are not required for the maintenance of the potentiation (not shown; electrical and chemical components averaged 122.4 and 206.7% of control values, respectively; n = 3).
Fig. 2.
Brief local application of DAMGO triggered a potentiation of the mixed synaptic response. A: local drug application in the vicinity of club ending contacts on the distal portion of the lateral dendrite. DAMGO (100 μM) was pressure ejected (Press.) with the help of a 2nd recording electrode (red arrow). The cartoon also illustrates the presence of dopaminergic fibers and varicosities in the vicinity of the club endings (Dopaminergic axonal terminations). (Diagram based on Fig. 5B of Pereda et al. 2004, used with permission from Elsevier.) B: local application of DAMGO triggered long-term potentiation of both components of the mixed synaptic response. DAMGO-evoked potentiation occluded activity-dependent potentiation evoked by the “100 Hz” stimulation protocol (blue bar, see material and methods for details), which is known to be mediated by endocannabinoids via local release of dopamine and requires activation of D1/5Rs (Cachope et al. 2007) Right: representative traces. C: example of the effect of the 100 Hz stimulation protocol on the amplitude of both components of the mixed synaptic response (unpublished single experiment from a previous experimental series) (Cachope et al. 2007).
DAMGO potentiation requires activation of dopamine D1/5 receptors.
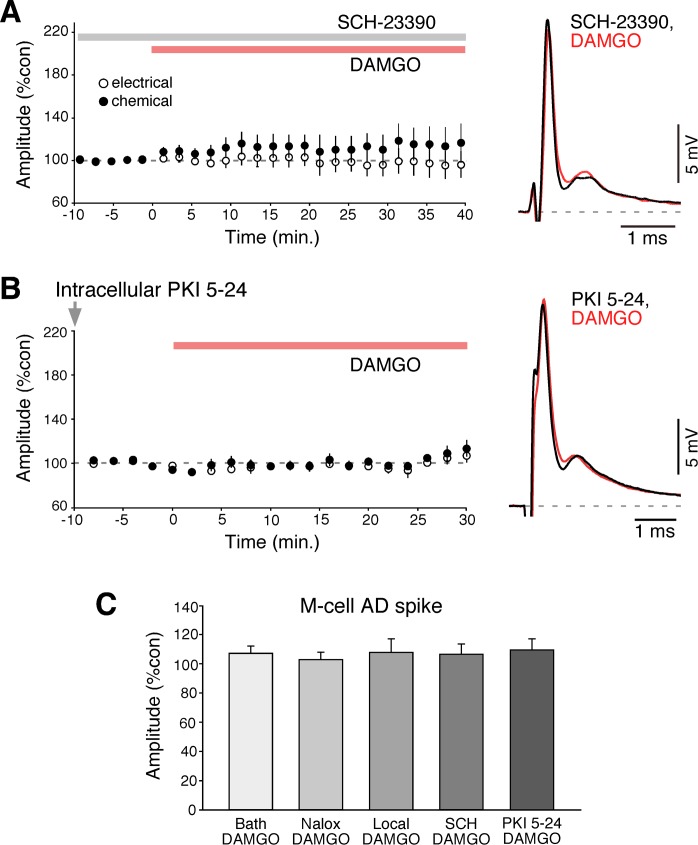
The effects of local DAMGO application were reminiscent of those observed by local application of agonists of cannabinoid type 1 receptor (CB1R), which also trigger long-term enhancements of both components of the synaptic response. Because opioids and endocannabinoids are known to converge for the regulation of multiple processes we asked if the potentiation triggered by local application of DAMGO could occlude the potentiation triggered by the “100 Hz protocol” (see material and methods for details). We have previously demonstrated that this activity protocol leads to a long-term potentiation of the mixed EPSP and that this potentiation is mediated by release of endocannabinoids from the M-cell, which via CB1R activation in turn leads to release of dopamine from dopaminergic fibers that are closely associated to the club endings and the M-cell dendrite (Cachope et al. 2007; Pereda et al. 1992) (Fig. 2A). As can be observed in Fig. 2B, local application of DAMGO occluded the 100 Hz potentiation since no further potentiation was observed in response to 100 Hz stimulation in any of the individual experiments (n = 5), suggesting that MORs and CB1Rs converge on a common regulatory mechanism. To explore this possibility, we asked if the potentiating effects of opioids were mediated via dopamine action by testing if DAMGO-evoked potentiation could be obtained in the presence of the D1/5 receptor antagonist SCH-23390 (50 μM) (Fig. 3A). We found that bath application of SCH-23390 blocked DAMGO effects; the mixed EPSP averaged 97.5 ± 12.9%, P > 0.8, and 115.6 ± 16.9%, P > 0.3, of control values for the electrical and chemical components, respectively (n = 6). Dopamine (Pereda et al. 1994) and CB1R-dependent potentiation (Cachope et al. 2007) are known to follow a cAMP-dependent pathway that requires activation of PKA. We reasoned that if DAMGO-evoked potentiation is mediated by dopamine, the potentiation should be prevented by PKA inhibitors. To test this possibility, the PKA inhibitory peptide PKI 5-24 was injected intradendritically prior to bath application of DAMGO; the mixed EPSP averaged 94 ± 5.89%, P > 0.1, and 95.7 ± 5.35%, P > 0.1, of control values for the electrical and chemical components, respectively (n = 5) (Fig. 3B). Together, these results suggest that DAMGO potentiation is likely to be mediated (as CB1R-mediated potentiation) via the release of dopamine from nearby dopaminergic fibers.
Fig. 3.
DAMGO-evoked potentiation requires activation of dopamine D1/5Rs. A: time course of both components of the mixed synaptic response for experiments in which bath application of DAMGO (red bar) followed pretreatment with the D1/5R antagonist SCH-23390 (gray bar, n = 5). B: time course for experiments in which the PKA inhibitor PKI 5-24 was applied intradendritically (gray arrow, 500 μM) prior to bath application of DAMGO (n = 5). C: neither bath or local application of drugs led to changes in the amplitude of the AD spike of the M-cell (an indicator of the M-cell input resistance), indicating that changes, when observed, were ascribable to modifications in the strength of electrical and chemical synapses. The M-cell AD spike averaged 107.4% after DAMGO application, 103.1% after Naloxone and DAMGO application, 111.2% after local DAMGO, 106.7% after SCH 23390 and DAMGO, and 109.9% after PKI 5-24 and DAMGO.
Importantly, neither bath nor local application of drugs led to changes in the amplitude of the AD spike of the M-cell (an indicator of the M-cell input resistance), indicating that changes, when observed, were ascribable to modifications of the synaptic strength (Fig. 3C). The M-cell AD spike averaged 107.4 ± 4.9% after DAMGO application, 103.1 ± 5.0% after Naloxone and DAMGO application, 111.2 ± 8.3% after local DAMGO, 106.7 ± 7.0% after SCH 23390 and DAMGO, and 109.9 ± 7.7% after PKI 5–24 and DAMGO.
Effects of opioids on the strength of inhibitory synapses.
We next investigated the effects of activation of MORs on inhibitory inputs to the M-cell. Because they contribute to a feedback inhibitory circuit (Fig. 4A), inhibitory terminals on the M-cell (GABAergic and glycinergic) can be activated by stimulating the M-cell axon antidromically. In contrast to glycinergic, GABAergic terminals are mostly distributed in the distal portion of the lateral dendrite (Triller et al. 1993) intermingled with club endings (Tuttle et al. 1987). This feedback or “recurrent” inhibition is generally hard to detect and measured as a synaptic potential because the equilibrium potential of chloride (Cl−) in the M-cell lies near to the resting membrane potential of the cell. A more accurate assay for the strength of these inhibitory synapses is to measure the change in membrane conductance produced by the opening of ligand-gated Cl− channels, detected as the decrease in the amplitude of a coincident AD spike. As mentioned previously, because the soma-dendritic membrane of the M-cell lacks active electrogenesis, the amplitude of the antidromic action potential represents a reliable estimate of this cell's resistance (see material and methods). To evidence changes in membrane conductance produced by inhibitory inputs, a second antidromic stimulating pulse was applied 6 ms after the first, conditioning, pulse (Fig. 4B). The change in membrane conductance, generally referred as fractional conductance, is proportional to the ratio between the amplitude of the M-cell antidromic spike in the absence (first spike) and presence of the inhibitory synaptic input (second spike; delivered at an interval that is coincident with the peak of the inhibitory conductance) (Oda et al. 1995). We asked whether this ratio could be affected by local application of DAMGO and found that this manipulation did not lead to changes in fractional conductance, averaging 99.9 ± 10.5%, P > 0.9 of its control values (n = 5) (Fig. 4C). In contrast, bath application of DAMGO, which in addition to the inhibitory terminals located in the dendrite near the club endings acts on inhibitory synaptic terminals located on proximal dendro-somatic regions, led to changes in fractional conductance that averaged 86.7 ± 4.3%, P < 0.05 of its control values (n = 8) (Fig. 4C), indicating a reduction in inhibitory strength. Consistent with these changes being mediated by opioids, no changes were observed in this ratio when DAMGO was applied in the presence of Naloxone and SCH-23390 (fractional conductance averaged 96.8 ± 5.1%, P > 0.5 of its control values, n = 5; and 98.0 ± 7.7%, P > 0.8 of its control values, n = 5, respectively) (Fig. 4C), indicating that changes of inhibitory strength promoted by DAMGO can be detected by this approach. Altogether, these findings suggest that (in contrast to those located in the proximal somatodendritic membrane) dendritic inhibitory terminals located nearby club endings are not the target of regulation by opioids in the M-cell system.
DISCUSSION
Our results indicate that both electrical and glutamatergic transmission at club endings become potentiated following activation of opioid receptors, indicating that the modulatory actions of endogenous and exogenous opioids are not restricted to chemical synapses but also include gap junction-mediated electrical synapses. While regulation of gap junctional communication by opioids was reported to occur at cardiomyocytes (Shi et al. 2012; Zhang et al. 2010), our results constitute the first evidence of their involvement in regulating electrical transmission in the vertebrate brain. Contrasting the involvement of kappa receptors in the heart (Shi et al. 2012), regulation of electrical transmission at club endings appears to require activation of MORs, which are known to be ubiquitous in the vertebrate central nervous system where they contribute to various physiological functions (Williams et al. 2001). Consistent with our findings, MORs with functional and pharmacological properties similar to those reported in mammals have been identified in zebrafish (Barrallo et al. 2000; Marron Fdez de Velasco et al. 2009), an ostariophysan teleost closely related to goldfish (Rupp et al. 1996). Moreover, behavioral experiments suggest the participation of opioid receptors in mediating nociception in goldfish (Ehrensing et al. 1982; Jansen and Greene 1970), which is thought to be mediated by MORs. We cannot exclude the possibility that some of the observed effects were also partially mediated by other types of opioid receptors, as the sensitivity of goldfish opioid isoforms for the agonist and antagonist used in our study remains unknown. Because gap junctions at club endings are formed by fish homologs of Cx36 (Pereda et al. 2003; Rash et al. 2013), a neuronal gap junction channel-forming protein that is widely distributed in the mammalian brain (Condorelli et al. 2000; Connors and Long 2004), the mechanisms of opioid regulation on electrical transmission described here might be widespread and specially relevant in structures of the reward system where opioids, endocannabinoids, and dopamine coexist (see below).
Opioid potentiation requires activation of dopamine receptors.
Contrasting the well-established action of opioids depressing chemical transmission (Williams et al. 2001), activation of opioid receptors triggered a long-term potentiation of both components of the mixed synaptic response. Our data indicate that opioids produced these effects by acting indirectly, via an already known potentiating mechanism that involves the participation of dopamine (Cachope et al. 2007; Pereda et al. 1992, 1994). Previous studies demonstrated 1) the presence of a rich dopaminergic innervation in the vicinity of the M-cell dendrite and the club endings, 2) that local application of dopamine was sufficient to trigger a long-term potentiation of both components of the mixed synaptic response, and 3) that this potentiation required activation of D1/5Rs and followed a PKA-dependent postsynaptic mechanism (Pereda et al. 1992, 1994). Supporting the involvement of this mechanism, DAMGO potentiation was blocked by both a D1/5R antagonist and intradendritic application of PKA blockers. Consistent with the effects of PKI 5-24, we have previously shown that the injection of the catalytic subunit of PKA triggers long-term potentiation of electrical (and chemical) transmission at these terminals (Pereda et al. 1994). Furthermore, we have also shown labeling of club endings with a phospho-antibody raised against the PKA phosphorylation site at serine 110 of Cx35 (anti-Cx35/P110) (Cachope et al. 2007). This dopamine-potentiating mechanism seems to be a converging property of several regulatory systems. Endocannabinoids released from the M-cell and locally applied CB1R agonists were shown to trigger a similar potentiation of the mixed EPSP by promoting the release of dopamine from varicosities located in the immediate vicinity of club endings (Cachope et al. 2007). The similarity of the effects of local activation of CB1Rs and MORs (see Fig. 2 and Cachope et al. 2007) and our findings that DAMGO-evoked potentiation occludes the “100 Hz” endocannabinoid-mediated potentiation that requires activation of CB1Rs and D1/5Rs (Cachope et al. 2007) suggests that opioids might follow a similar mechanism, regulating the local availability of dopamine. Opioids were reported to increase dopamine availability by depressing synaptic release at inhibitory interneurons that slow down the activity of dopaminergic neurons, a phenomenon known as “disinhibition” (Olds 1982; Williams et al. 2001). The absence of effect of DAMGO on the strength of inhibitory synapses on the M-cell dendrite indicates that this mechanism is unlikely to be responsible for the local increase in dopamine necessary to modify mixed transmission.
It was proposed that activation of CB1Rs located on dopaminergic varicosities could lead to the release of dopamine via a nonconventional intracellular signaling pathway (Cachope et al. 2007). This possibility was supported by 1) the presence of punctate labeling for CB1Rs associated to dopaminergic varicosities and 2) lack of evidence for disinhibition, as inhibitory inputs to the lateral dendrite were not affected by cannabinoids (Cachope et al. 2007). Since inhibitory inputs in the dendrite were similarly unaffected by local application of DAMGO we propose that MORs could act similarly. Supporting this possibility, opioid receptors were reported to be present on axons and varicosities containing various modulatory neurotransmitters (Drake and Milner 1999; Milner and Drake 2001). Unfortunately, we could not explore the association of MORs to dopaminergic varicosities due to the lack of antibodies recognizing fish MORs, as teleost orthologs for these receptors lack the sequence for the epitope at which most available antibodies were targeted (Barrallo et al. 2000). Nevertheless, based on the striking mechanistic similarities between MOR and CB1R potentiations we propose that both endocannabinoids and opioids converge for the regulation of the availability of dopamine in the M-cell lateral dendrite (Fig. 5) by promoting its release from dopaminergic fibers and varicosities. We predict that other, currently unknown, modulatory neurotransmitters could similarly regulate the local availability of dopamine.
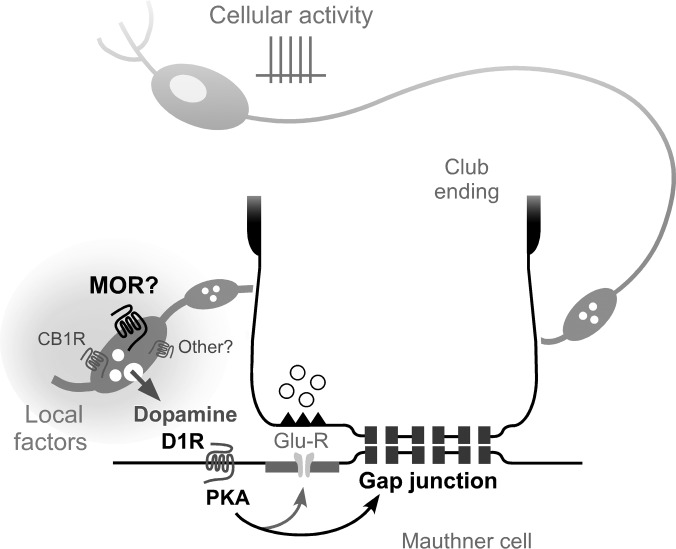
Fig. 5.
Local factors determine the availability of dopamine in the vicinity of club endings. Proposed model: mu-opioid receptors (MORs) could be located [as previously proposed for cannabinoid type 1 receptors (CB1Rs)] on dopaminergic fibers and varicosities located in the immediate vicinity of the club endings and the M-cell lateral dendrite. Release of dopamine triggers a long-term potentiation of the mixed synaptic response via a PKA-dependent postsynaptic mechanism that requires activation of D1/5Rs (D1R). Both locally released opioids and endocannabinoids, and possibly other neurotransmitter modulators (other?), converge for the regulation of the local availability of dopamine by triggering its release. In addition to the activity of dopaminergic neurons (cellular activity) whose somata are located remotely, locally generated factors can control the availability of dopamine in the vicinity of the club endings in an independent manner.
Local control of dopamine availability.
Our data suggest that the availability of dopamine in the M-cell dendrite could be increased by either 1) the activity of dopaminergic neurons themselves, whose cell bodies are located remotely, or 2) local release of regulatory molecules acting in a paracrine fashion (Fig. 5). The coexistence of these two modalities of dopamine release is supported by evidence obtained in mammals. Local pharmacological activation of opioid receptors in the ventral tegmental area is known to enhance the release of dopamine at the nucleus accumbens, where ventral tegmental area axons project, by depressing synaptic transmission at inhibitory terminals on ventral tegmental area dopaminergic neurons (disinhibition). However, application of opioid receptor agonists at the nucleus accumbens can also regulate dopamine availability in this nucleus (Olds 1982), suggesting that not only somata but also axonic terminations of ventral tegmental area dopaminergic cells are targets for opioid regulation (Olds 1982). Moreover, recent evidence suggests that other neurotransmitters can act on axonal terminations of dopaminergic neurons to trigger release. That is, acetylcholine was shown to directly act on dopaminergic terminals to increase dopamine availability in the nucleus accumbens (Cachope et al. 2012). Thus, control of dopamine availability by local factors might represent a widespread mechanism. While the activity of dopaminergic neurons allows a simultaneous increase in dopamine at multiple targets supporting behaviors that require the concerted activation of multiples brain structures, local factors could administer dopamine actions only in the involved structure.
Convergence of multiple mechanisms of synaptic potentiation at Club endings.
Club endings are terminations of primary auditory afferents with anatomical and functional specializations that make them ideal candidates to provide the M-cell with the essential information for the initiation of an escape response (reviewed in Curti and Pereda 2010). Perhaps the most remarkable specialization of club ending afferents is the ability of their synapses to undergo activity-dependent potentiation of both electrical chemical and synapses either via activation of NMDA receptors (Pereda and Faber 1996; Yang et al. 1990) or involving the release of endocannabinoids (Cachope et al. 2007). These plastic properties are likely to represent a type of sensory-motor processing that leads to the sensitization of the escape response (Yang et al. 1990). Adding to these two mechanisms, our results show that club endings are likely also under the regulatory control of the endogenous opioid system whose involvement also leads to potentiation of these synapses. The source of production and identity of the endogenous opioid responsible for the effects as well as the physiological conditions under which it is released requires further investigation and exceeds the scope of this article. A provocative possibility would be that opioids and endocannabinoids are, as in hypothalamic neurons (Iremonger and Bains 2009; Iremonger et al. 2011), both released from the M-cell lateral dendrite.
Interestingly, it was reported that local application of somatostatin, a peptide that was estimated to be present in 80% of club endings (Sur et al. 1994), triggers long-term potentiation of electrical and chemical transmission at these terminals (Pereda et al. 1997). Although the involved cellular mechanisms remain unknown, the evidence suggests the existence of a fourth form of potentiation that requires the release of somatostatin from club endings likely as a result of specific patterns of presynaptic activity. Moreover, this finding opens the possibility that other, currently unknown, mechanisms of potentiation are also present at these terminals.
From an engineering perspective, such striking coincidence of mechanism of potentiation could be interpreted as redundant mechanisms that coexist for fail-safety in the regulation of the escape response. An alternative, more biological, interpretation would be that this phenomenon reflects the evolution-driven convergence of several neurotransmitter systems with different overall functions on the regulation of a behaviorally relevant cellular property. One could speculate that NMDAR-mediated potentiation of auditory mixed synapses evolved to underlie experience-dependent sensitization, whereas opioids, which derive from a primitive anti-inflammatory molecule (Stefano and Kream 2008), could act to enhance escape as part of an organism-wide response to injury (which includes pain control). Potentiation of these synapses could also be related to the well-established roles of endocannabinoids in motor learning (Heifets and Castillo 2009) and growth and survival of somatostatin (Reisine 1995). We suggest that from the biological point of view, the term “degeneracy” is more adequate than “redundancy” to illustrate this phenomenon (Edelman and Gally 2001). As opposed to redundancy (typical of engineering design) where the same function is performed by identical systems, degeneracy refers to the ability of systems that are structurally different to perform the same “function.” In the case of the M-cell network the function is the potentiation of a behaviorally relevant synaptic input. It has been proposed that degeneracy of biological networks is a major contributor to their complexity, allowing widespread compensatory homeostatic adjustments (Edelman and Gally 2001; Whitacre 2010). Similarly, degeneracy of mechanisms of synaptic plasticity might constitute a property of key synaptic contacts within a neural network and an expression of its complexity and robustness (Whitacre 2010; Gutierrez and Marder 2013).
GRANTS
Supported by the Grass Foundation and National Institutes of Health Grants DC-03186, DC-011099, and NS-0552827 (to A. E. Pereda).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.C. and A.E.P. conception and design of research; R.C. and A.E.P. performed experiments; R.C. and A.E.P. analyzed data; R.C. and A.E.P. interpreted results of experiments; R.C. and A.E.P. prepared figures; R.C. and A.E.P. drafted manuscript; R.C. and A.E.P. edited and revised manuscript; R.C. and A.E.P. approved final version of manuscript.
ACKNOWLEDGMENTS
Current address for R. Cachope: CHDI Foundation, 6080 Center Dr., Ste. 100, Los Angeles, CA 90045.
REFERENCES
- Allison DW, Ohran AJ, Stobbs SH, Mameli M, Valenzuela CF, Sudweeks SN, Ray AP, Henriksen SJ, Steffensen SC. Connexin-36 gap junctions mediate electrical coupling between ventral tegmental area GABA neurons. Synapse 60: 20–31, 2006. [DOI] [PubMed] [Google Scholar]
- Barrallo A, González-Sarmiento R, Alvar F, Rodríguez RE. ZFOR2, a new opioid receptor-like gene from the teleost zebrafish (Danio rerio). Mol Brain Res 84: 1–6, 2000. [DOI] [PubMed] [Google Scholar]
- Bartelmez GW, Hoerr NL. The vestibular club endings in ameiurus. Further evidence on the morphology of the synapse. J Comp Neurol 57: 401–428, 1933. [Google Scholar]
- Bennett MVL, Zukin RS. Electrical coupling and neuronal synchronization in the mammalian brain. Neuron 41: 495–511, 2004. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Völgyi B. The diverse functional roles and regulation of neuronal gap junctions in the retina. Nat Rev Neurosci 10: 495–506, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundege JM, Williams JT. Differential modulation of nucleus accumbens synapses. J Neurophysiol 88: 142–151, 2002. [DOI] [PubMed] [Google Scholar]
- Cachope R, Mackie K, Triller A, O'Brien J, Pereda AE. Potentiation of electrical and chemical synaptic transmission mediated by endocannabinoids. Neuron 56: 1034–1047, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachope R, Mateo Y, Mathur BN, Irving J, Wang HL, Morales M, Lovinger DM, Cheer JF. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep 2: 33–41, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers SR. Opioid receptor-coupled second messenger systems. Life Sci 48: 1991–2003, 1991. [DOI] [PubMed] [Google Scholar]
- Condorelli DF, Belluardo N, Trovato-Salinaro A, Mudò G. Expression of Cx36 in mammalian neurons. Brain Res Brain Res Rev 32: 72–85, 2000. [DOI] [PubMed] [Google Scholar]
- Condorelli DF, Parenti R, Spinella F, Trovato Salinaro A, Belluardo N, Cardile V, Cicirata F. Cloning of a new gap junction gene (Cx36) highly expressed in mammalian brain neurons. Eur J Neurosci 10: 1202–1208, 1998. [DOI] [PubMed] [Google Scholar]
- Connors BW, Long MA. Electrical synapses in the mammalian brain. Annu Rev Neurosci 27: 393–418, 2004. [DOI] [PubMed] [Google Scholar]
- Curti S, Pereda AE. Functional specializations of primary auditory afferents on the Mauthner cells: interactions between membrane and synaptic properties. J Physiol (Paris) 104: 203–214, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CT, Milner TA. Mu opioid receptors are in somatodendritic and axonal compartments of GABAergic neurons in rat hippocampal formation. Brain Res 849: 203–215, 1999. [DOI] [PubMed] [Google Scholar]
- Dreborg S, Sundström G, Larsson TA, Larhammar D. Evolution of vertebrate opioid receptors. Proc Natl Acad Sci USA 105: 15487–15492, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman GM, Gally JA. Degeneracy and complexity in biological systems. Proc Natl Acad Sci USA 98: 13763–13768, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrensing RH, Michell GF, Kastin AJ. Similar antagonism of morphine analgesia by MIF-1 and naloxone in Carassius auratus. Pharmacol Biochem Behav 17: 757–761, 1982. [DOI] [PubMed] [Google Scholar]
- Faber DS, Pereda AE. Physiology of the Mauthner Cell: Function. Elsevier, 2011. [Google Scholar]
- Fukami Y, Furukawa T, Asada Y. Excitability changes of the Mauthner cell during collateral inhibition. J Gen Physiol 48: 581–600, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furshpan EJ. “Electrical transmission” at an excitatory synapse in a vertebrate brain. Science 144: 878–880, 1964. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Furshpan EJ. Two inhibitory mechanisms in the Mauthner neurons of goldfish. J Neurophysiol 26: 140–176, 1963. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Paul DL. Gap junctions. Cold Spring Harb Perspect Biol 1: a002576, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez GJ, Marder E. Rectifying electrical synapses can affect the influence of synaptic modulation on output pattern robustness. J Neurosci 33: 13238–13248, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifets BD, Castillo PE. Endocannabinoid signaling and long-term synaptic plasticity. Annu Rev Physiol 71: 283–306, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iremonger KJ, Bains JS. Retrograde opioid signaling regulates glutamatergic transmission in the hypothalamus. J Neurosci 29: 7349–7358, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iremonger KJ, Kuzmiski JB, Baimoukhametova DV, Bains JS. Dual regulation of anterograde and retrograde transmission by endocannabinoids. J Neurosci 31: 12011–12020, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen GA, Greene NM. Morphine metabolism and morphine tolerance in goldfish. Anesthesiology 32: 231–235, 1970. [DOI] [PubMed] [Google Scholar]
- Lin JW, Faber DS. Synaptic transmission mediated by single club endings on the goldfish Mauthner cell. II. Plasticity of excitatory postsynaptic potentials. J Neurosci 8: 1313–1325, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni OJ, Williams JT. Presynaptic regulation of glutamate release in the ventral tegmental area during morphine withdrawal. J Neurosci 19: 6629–6636, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marron Fdez de Velasco E, Law PY, Rodríguez RE. Mu opioid receptor from the zebrafish exhibits functional characteristics as those of mammalian mu opioid receptor. Zebrafish 6: 259–268, 2009. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dollé P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature 383: 819–823, 1996. [DOI] [PubMed] [Google Scholar]
- Milner TA, Drake CT. Ultrastructural evidence for presynaptic mu opioid receptor modulation of synaptic plasticity in NMDA-receptor-containing dendrites in the dentate gyrus. Brain Res Bull 54: 131–140, 2001. [DOI] [PubMed] [Google Scholar]
- O'Brien J. The ever-changing electrical synapse. Curr Opin Neurobiol 29C: 64–72, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P, Grace AA. Dopaminergic modulation of dye coupling between neurons in the core and shell regions of the nucleus accumbens. J Neurosci 13: 3456–3471, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Charpier S, Murayama Y, Suma C, Korn H. Long-term potentiation of glycinergic inhibitory synaptic transmission. J Neurophysiol 74: 1056–1074, 1995. [DOI] [PubMed] [Google Scholar]
- Olds ME. Reinforcing effects of morphine in the nucleus accumbens. Brain Res 237: 429–440, 1982. [DOI] [PubMed] [Google Scholar]
- Oleskevich S, Clements JD, Williams JT. Opioid-glutamate interactions in rat locus coeruleus neurons. J Neurophysiol 70: 931–937, 1993. [DOI] [PubMed] [Google Scholar]
- Onn SP, Grace AA. Amphetamine withdrawal alters bistable states and cellular coupling in rat prefrontal cortex and nucleus accumbens neurons recorded in vivo. J Neurosci 20: 2332–2345, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda A, O'Brien J, Nagy JI, Bukauskas F, Davidson KGV, Kamasawa N, Yasumura T, Rash JE. Connexin35 mediates electrical transmission at mixed synapses on Mauthner cells. J Neurosci 23: 7489–7503, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda A, Reisine T, Faber DS, Korn H. Somatostatin enhances excitatory synaptic transmission at mixed synapses on the Mauthner (M-) cell. In: Society for Neurosciences Abstracts. 1997, p. 1180.
- Pereda A, Triller A, Korn H, Faber DS. Dopamine enhances both electrotonic coupling and chemical excitatory postsynaptic potentials at mixed synapses. Proc Natl Acad Sci USA 89: 12088–12092, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda AE, Curti S, Hoge G, Cachope R, Flores CE, Rash JE. Gap junction-mediated electrical transmission: regulatory mechanisms and plasticity. Biochim Biophys Acta 1828: 134–146, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda AE, Faber DS. Activity-dependent short-term enhancement of intercellular coupling. J Neurosci 16: 983–992, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda AE, Nairn AC, Wolszon LR, Faber DS. Postsynaptic modulation of synaptic efficacy at mixed synapses on the Mauthner cell. J Neurosci 14: 3704–3712, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda AE, Rash JE, Nagy JI, Bennett MVL. Dynamics of electrical transmission at club endings on the Mauthner cells. Brain Res Brain Res Rev 47: 227–244, 2004. [DOI] [PubMed] [Google Scholar]
- Pereda AE. Electrical synapses and their functional interactions with chemical synapses. Nat Rev Neurosci 15: 250–263, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Curti S, Vanderpool KG, Kamasawa N, Nannapaneni S, Palacios-Prado N, Flores CE, Yasumura T, O'Brien J, Lynn BD, Bukauskas FF, Nagy JI, Pereda AE. Molecular and functional asymmetry at a vertebrate electrical synapse. Neuron 79: 957–969, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisine T. Somatostatin. Cell Mol Neurobiol 15: 597–614, 1995. [DOI] [PubMed] [Google Scholar]
- Robertson JD, Bodenheimer TS, Stage DE. The ultrastructure of Mauthner cell synapses and nodes in goldfish brains. J Cell Biol 19: 159–199, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp B, Wullimann MF, Reichert H. The zebrafish brain: a neuroanatomical comparison with the goldfish. Anat Embryol (Berl) 194: 187–203, 1996. [DOI] [PubMed] [Google Scholar]
- Shi QX, Zhang LJ, Yao Y, Zhang QY, Wang W, Li J, Shang YL, Bi H, Zhang SM, Guo HT, Wang YM, Yu SQ, Yi DH, Bueno FR, Kaye AD, Pei JM. κ-opioid receptor activation prevents against arrhythmias by preserving Cx43 protein via alleviation of intracellular calcium. Am J Ther 20: 493–501, 2013. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Stopfer M. Gap junctions. Curr Biol 23: R1026–R1031, 2013. [DOI] [PubMed] [Google Scholar]
- Stefano GB, Kream R. Endogenous opiates, opioids, and immune function: evolutionary brokerage of defensive behaviors. Semin Cancer Biol 18: 190–198, 2008. [DOI] [PubMed] [Google Scholar]
- Stevens CW. The evolution of vertebrate opioid receptors. Front Biosci (Landmark Ed) 14: 1247–1269, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JD, Liu Y, Yuan YH, Li J, Chen NH. Gap junction dysfunction in the prefrontal cortex induces depressive-like behaviors in rats. Neuropsychopharmacology 37: 1305–1320, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Korn H, Triller A. Colocalization of somatostatin with GABA or glutamate in distinct afferent terminals presynaptic to the Mauthner cell. J Neurosci 14: 576–589, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triller A, Sur C, Korn H. Heterogeneous distribution of glycinergic and GABAergic afferents on an identified central neuron. J Comp Neurol 338: 83–96, 1993. [DOI] [PubMed] [Google Scholar]
- Tuttle R, Masuko S, Nakajima Y. Small vesicle bouton synapses on the distal half of the lateral dendrite of the goldfish Mauthner cell: freeze-fracture and thin section study. J Comp Neurol 265: 254–274, 1987. [DOI] [PubMed] [Google Scholar]
- Vandecasteele M, Deniau JM, Glowinski J, Venance L. Electrical synapses in basal ganglia. Rev Neurosci 18: 15–35, 2007. [DOI] [PubMed] [Google Scholar]
- Vandecasteele M, Glowinski J, Venance L. Electrical synapses between dopaminergic neurons of the substantia nigra pars compacta. J Neurosci 25: 291–28, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraraghavan R, Gourdie RG, Poelzing S. Mechanisms of cardiac conduction: a history of revisions. Am J Physiol Heart Circ Physiol 306: H619–H627, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venance L, Glowinski J, Giaume C. Electrical and chemical transmission between striatal GABAergic output neurones in rat brain slices. J Physiol 559: 215–230, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitacre JM. Degeneracy: a link between evolvability, robustness and complexity in biological systems. Theor Biol Med Model 7: 6, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev 81: 299–343, 2001. [DOI] [PubMed] [Google Scholar]
- Yang XD, Korn H, Faber DS. Long-term potentiation of electrotonic coupling at mixed synapses. Nature 348: 542–545, 1990. [DOI] [PubMed] [Google Scholar]
- Zhang QY, Wang W, Shi QX, Li YL, Huang JH, Yao Y, Li J, Zhang SM, Fan R, Zhou JJ, Guo HT, Wang YM, Yin W, Pei JM. Antiarrhythmic effect mediated by κ-opioid receptor is associated with Cx43 stabilization. Crit Care Med 38: 2365–2376, 2010. [DOI] [PubMed] [Google Scholar]