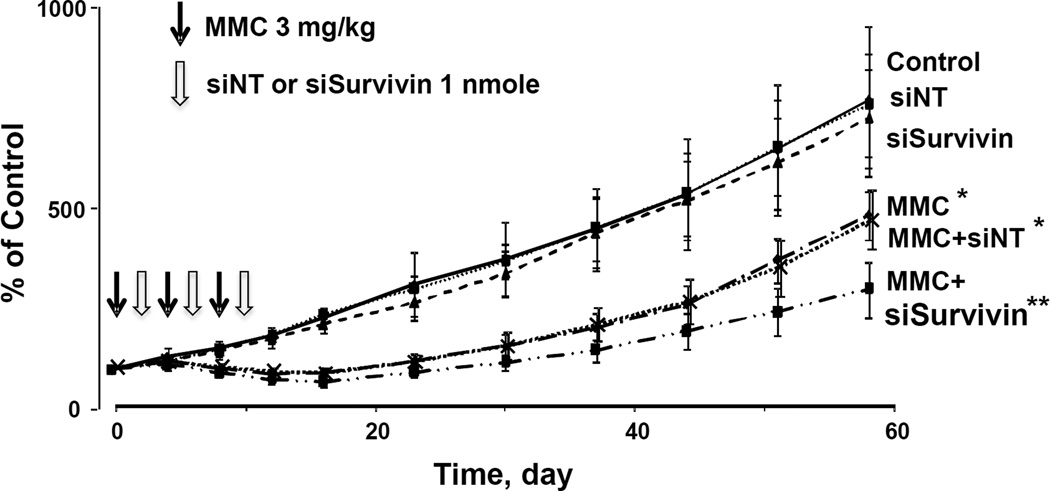
Figure 3. Survivin silencing enhances antitumor activity of MMC in vivo: Tumor growth.
Antitumor activity was evaluated in immunodeficient mice bearing subcutaneous RT4 human bladder xenograft tumors. All treatments (indicated by arrows) were administered by intravenous injections. Day 0 represents the day of treatment initiation, which corresponded to 28 days post-tumor implantation. The MMC dose was 3 mg/kg, given every 4 days for a total of 3 doses. The PPCat-siSurvivin dose was 1 nmole, given 2 days after each MMC treatment. Animals were maintained for 58 days post treatment, with tumor size measurements taken every 4 days for the first 16 days and once a week afterwards. From top to bottom: control (filled diamonds, n=5), siNT (open squares, n=5), siSurvivin (filled triangles, n=5), MMC (open diamonds, n=10), MMC+siNT (crosses, n=5), MMC+siSurvivin (filled squares, n=10). *p<0.05 vs. control and single agent siNT and siSurvivin. **p<0.05 vs. all other groups.