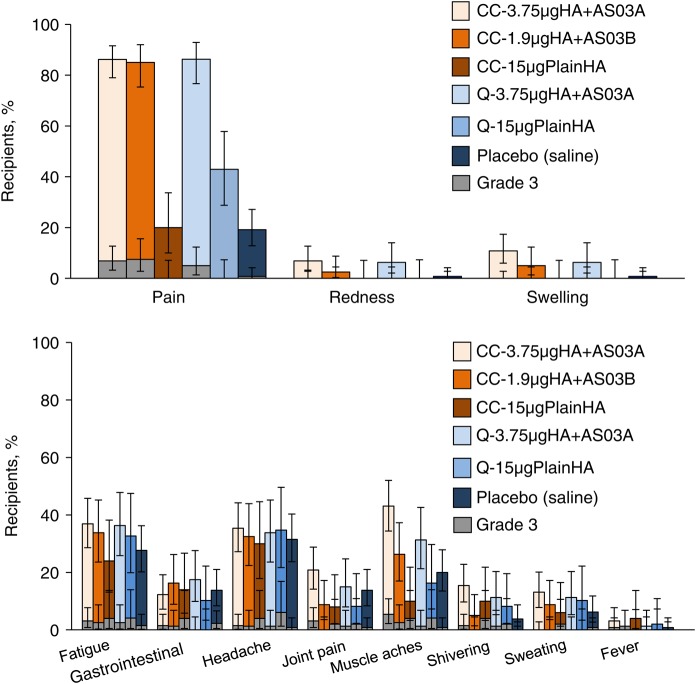
Figure 2.
Percentage of subjects reporting solicited injection site or general symptoms within 7 days of either vaccine dose—total vaccinated cohort. Vertical lines indicate 95% confidence intervals. Grade 3 is defined as redness and swelling (diameter, >100 mm), a temperature of ≥39.0°C (any route), and, for all other symptoms, as symptoms with sufficient severity to prevent normal activities (eg inability to attend work/school). CC-1.9 µgHA + AS03B, cell-culture-derived formulation with 1.9 µg of HA plus AS03B adjuvant; CC-3.75 µgHA + AS03A, cell-culture-derived formulation with 3.75 µg of HA plus AS03A adjuvant; CC-15 µgPlainHA, cell-culture-derived formulation with 15 µg of HA without adjuvant; HA, hemagglutinin antigen; Q-3.75 µgHA + AS03A, egg-derived formulation with 3.75 µg of HA plus AS03A adjuvant; Q-15 µgPlainHA, egg-derived formulation with 15 µg of HA without adjuvant.