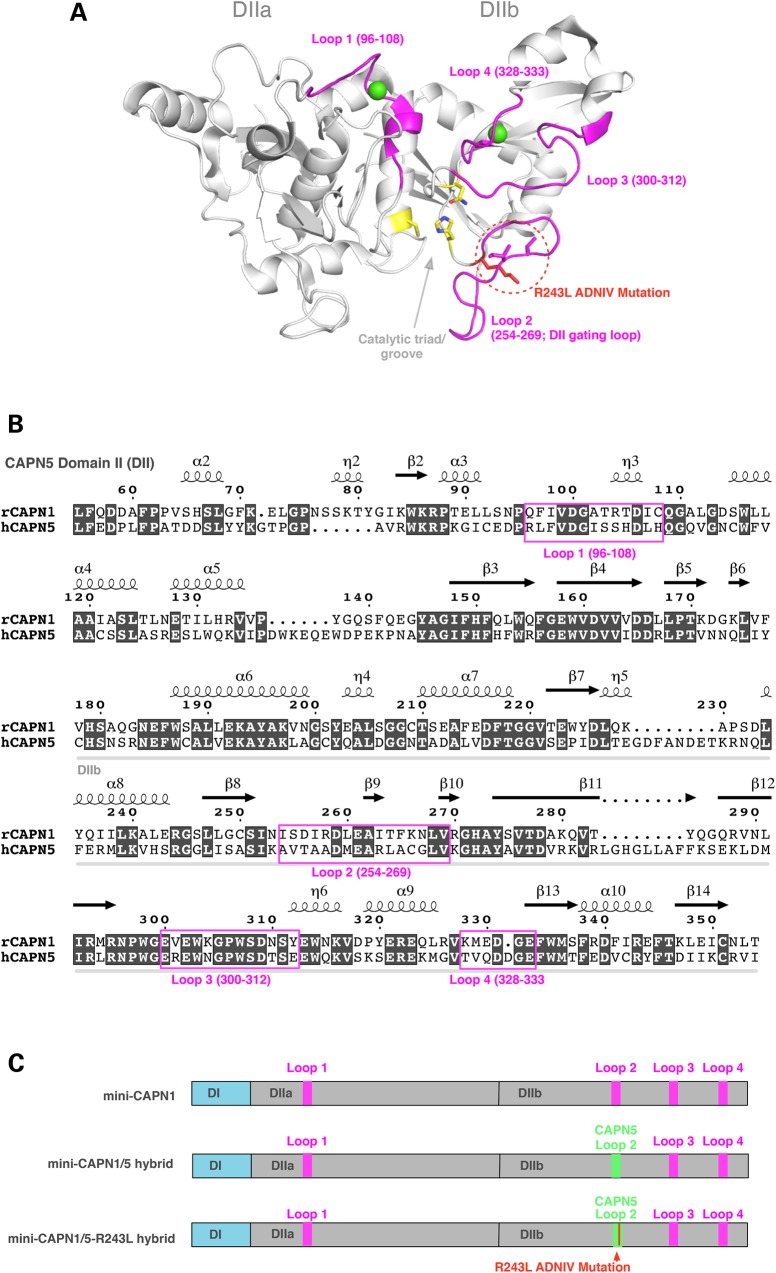
Figure 1.
Generation of a recombinant mini-CAPN1/5 hybrid with loop 2 of proteolytic domain IIb (DIIb) swapped from CAPN5 into the homologous region in mini-CAPN1. The mini-calpain system allows the catalytic activity of domain II to be isolated and assayed, since full-length calpains are generally not stable. (A) Cartoon representation of the rat mini-CAPN5 structure with calcium bound (green spheres), highlighting the active site residues (yellow sticks), the four flexible loops (pink) and the ADNIV mutations (red dotted circle). (B) Sequence alignment of domain II of rat mini-CAPN1 and human mini-CAPN5 highlighting the flexible loops (pink box). (C) Schematic representation showing proteins used in our modified mini-calpain system. Mini-CAPN1, a hybrid mini-CAPN1/5 with loop 2 of mini-CAPN1 swapped out with that of mini-CAPN5 and a hybrid mini-CAPN1/5 harboring the p.R243L ADNIV mutation.