Figure 3. Mechanism and scope of Au-catalyzed benzannulation of siloxy alkyne with 2-pyrones.
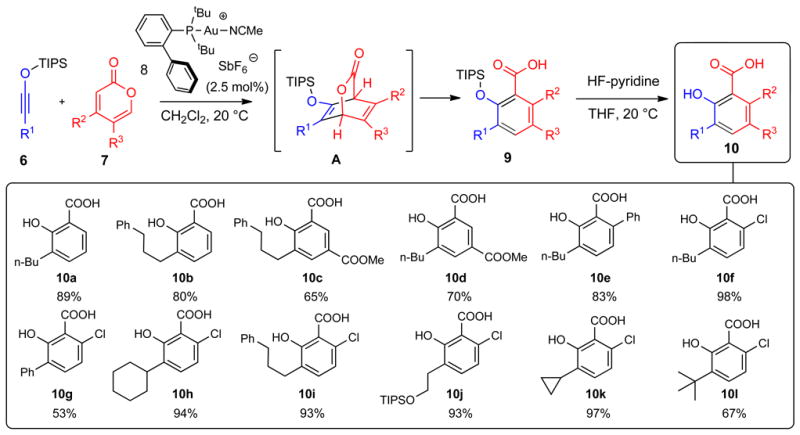
Siloxy alkyne 6 undergoes a [4+2] cycloaddition with 2-pyrone 7 to give a putative intermediate A, which undergoes subsequent fragmentation to deliver carboxylic acid 9. Compound numbers are shown in bold. Isolated yields are shown below each compound number. R is a generic alkyl or aryl substituent.
