Figure 4. Mechanism and scope of Au-catalyzed benzannulation siloxy alkynes with isoquinoline N-oxides.
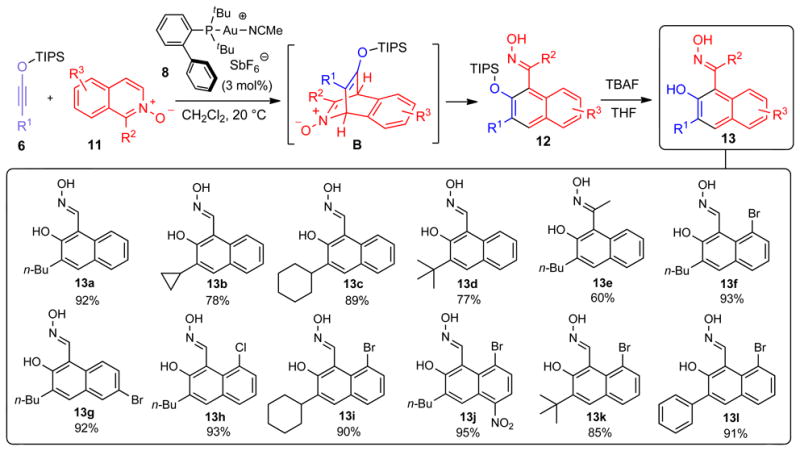
Siloxy alkyne 6 undergoes a [4+2] cycloaddition with isoquinoline N-oxide 11 to give a putative intermediate B, which undergoes subsequent fragmentation to deliver oxime 12. Compound numbers are shown in bold. Isolated yields are shown below each compound number. R is a generic alkyl or aryl substituent. Due to instability, the yield of compound 13h was determined by NMR using an internal standard. The product of this reaction was subsequently isolated as the corresponding imine in 82% yield following treatment of 13h with MoCl5 and Zn in acetonitrile as described in Supplementary Information.
