Fig. 2.
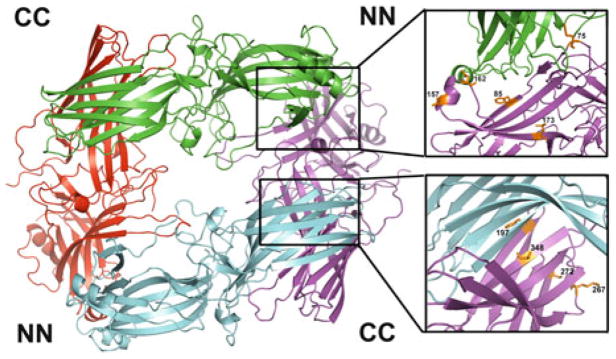
Solution tetramer of arrestin-1. Studies using site-directed spin labeling EPR, long-range inter-subunit distance measurements by DEER spectroscopy, site-directed mutagenesis, Rosetta modeling, and inter-subunit disulfide bridge formation (Hanson et al. 2007c, 2008a) lead to the conclusion that the solution tetramer of arrestin-1 is a symmetrical closed diamond, where adjacent protomers interact via two types of interfaces: C-to-C domain (CC) and N-to-N domain (NN). Enlarged interfaces are shown on the right, with residues in positions experimentally tested by various methods shown as stick models (see text for details)
