Figure 4. The rapamycin-binding site of the FRB recruits S6K1 into the catalytic cleft.
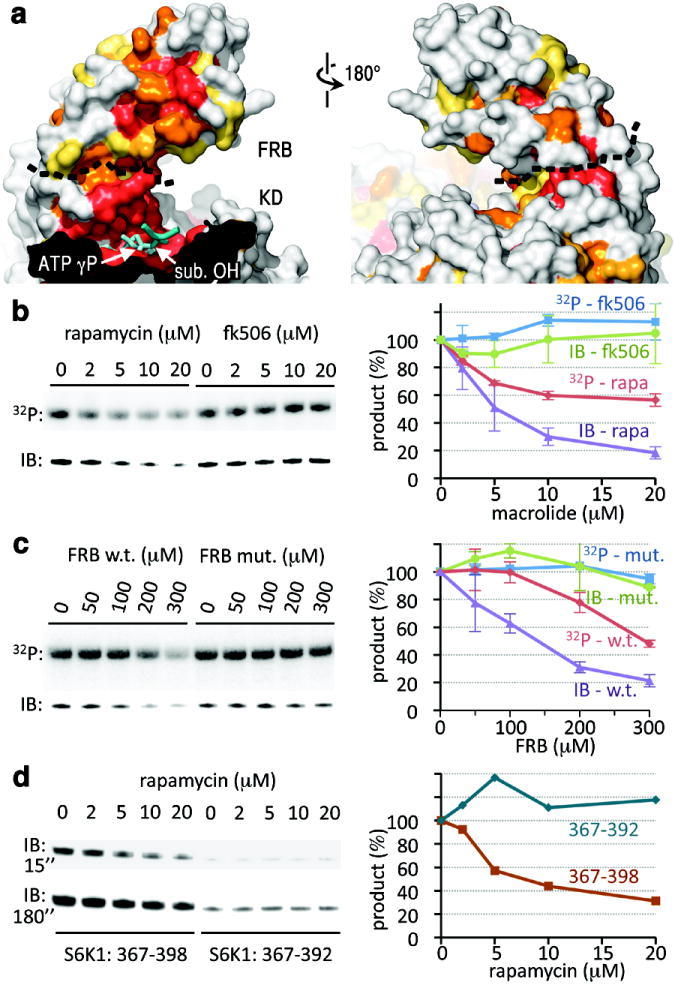
a, Surface representations of the FRB and portion of the catalytic cleft, coloured by sequence conservation as in Figure 2d. ADP-MgF3-Mg2 is in cyan with the TS mimic MgF3 group labelled “ATP γP”. The black dashed line delineates the FRB-KD boundary. The docked substrate peptide from Figure 2d is shown in blue (positions -1 to +1), with its phosphorylation site labelled “sub. OH”.
b, Phosphorylation of S6K1ki (10 μM) by mTORΔN-mLST8 (20 nM), measured by 32P incorporation (top panel) and by immunoblotting with a phosphoThr389-specific antibody (lower panel), in the presence of the indicated concentrations of rapamycin or FK506. The average and standard deviation of three independent repetitions is plotted as a percentage of the 0 μM macrolide reaction of each set.
c, Phosphorylation of S6K1ki (10 μM) by mTORΔN-mLST8 (20 nM) in the presence of the indicated concentrations of the wild type or S2035I mutant FRB. Reactions were repeated twice, and results plotted as in (b).
d, Phosphorylation of GST-tagged S6K1367-398 (10 μM) and S6K1367-392 (10 μM) by mTORΔN-mLST8 (20 nM) in the presence of the indicated concentrations of rapamycin. Two different exposures (15 and 180 sec.) of the phosphoThr389-specific immunoblot are shown. The quantitation of the 15 sec. immunoblot is plotted on the right.
