Figure 1.
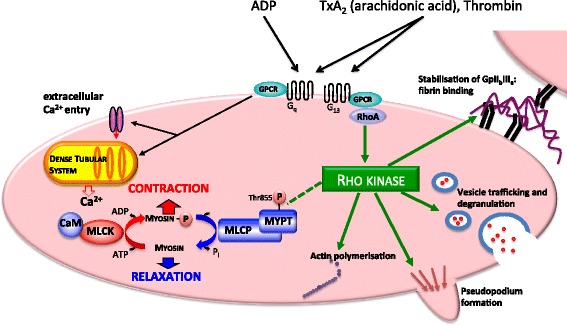
Platelet aggregation requires contraction, adhesion and secretion. ROK promotes platelet aggregation independent of MLCP inhibition. Platelet contraction requires Ser19 myosin light chain (MLC20) phosphorylation by Ca2+:calmodulin (CaM)-dependent myosin light chain kinase (MLCK). Agonists such as arachidonic acid initiate platelet contraction by augmenting [Ca2+]cyt derived from both the extracellular fluid via transient receptor potential (TRP) channels and intracellular dense tubular system stores. Contraction is opposed when MLC20 is dephosphorylated by myosin light chain phosphatase (MLCP). It is proposed that MLCP is regulated by two mechanisms: (i) G protein-coupled receptors (GPCRs) bearing the Gα13 subunit activate membrane-bound RhoA, which activates Rho kinase (ROK) [23]; ROK is thought to inhibit MLCP by phosphorylating Thr855 on MYPT, the regulatory subunit of MLCP [24-26] (dashed line). (ii) PKC-dependent CPI-17 directly inhibits MLCP [24,27-29] (not shown). By inhibiting MLCP, both ROK and CPI-17 favour contraction independent of the prevailing [Ca2+]. Other than disinhibiting MLCP, there are other mechanisms by which ROK inhibition might oppose platelet aggregation: ROK is also involved in vesicle trafficking and degranulation, pseudopodium formation, stabilisation of platelet-fibrin binding and actin polymerisation.
