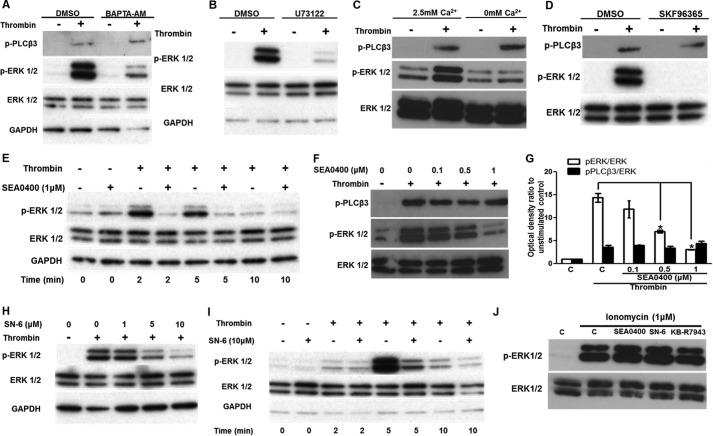
FIGURE 1.
Ca2+ influx through reverse-mode NCX is required for thrombin-induced ERK1/2 activation. A, HUVECs, serum-starved for 40 min, were treated with BAPTA-AM (10 μm) or vehicle for a further 20 min prior to challenge with thrombin (0.5 units/ml) for 5 min. ERK1/2 and PLCβ3 activation were demonstrated by Western blot. Membranes were subsequently stripped and re-probed for total ERK1/2 and GAPDH proteins. B, serum-starved HUVECs were preincubated with the PLC inhibitor U73122 (1 μm) for 30 min prior to challenge with thrombin (0.5 units/ml) for 5 min. C, HUVECs were serum-starved for 45 min and then incubated for a further 15 min in Ca2+-free medium, prior to challenge with 0.5 units/ml thrombin (5 min). ERK1/2 and PLCβ3 activation was determined as in A. D, HUVECs were incubated with the broad-spectrum inhibitor of nonselective cation channels SKF96365 (30 μm) and treated as in B. E, serum-starved HUVECs were preincubated with the reverse-mode NCX inhibitor SEA0400 (1 μm) or vehicle for 30 min prior to challenge with thrombin (0.5 units/ml) for the times indicated. F, SEA0400 was also applied over a range of concentrations, and ERK1/2 and PLCβ3 activation was assayed after 5 min. G, optical densities of the phospho-ERK1/2 and phospho-PLCβ3 bands in F were normalized against the ERK1/2 total protein of the corresponding sample. The normalized density of the unstimulated control (bar C) was set to 1. The mean value of the ratio for each condition is expressed as fold × unstimulated control value. Bars represent the means ± S.E. from n = 3 experiments. *, p < 0.05 versus the thrombin-stimulated control. A second reverse-mode NCX inhibitor, SN-6, also suppressed thrombin-induced ERK1/2 activation in a dose-dependent (H) and time-dependent manner (I). HUVECs were stimulated with ionomycin (1 μm for 5 min) in the presence of SEA0400 (1 μm), SN-6 (10 μm), KB-R7943 (10 μm), or vehicle (J), and ERK1/2 activation was assessed by Western blot. n = 3.