FIGURE 3.
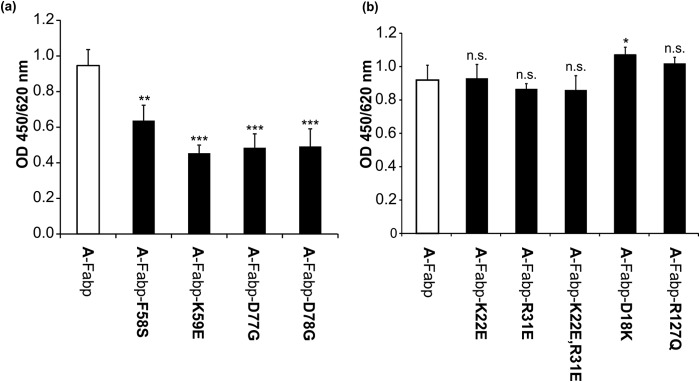
Binding of Cgi-58 to various mutant A-Fabp variants. a, in solid phase assays, polystyrene plates were coated with 3 μg of purified wild type A-Fabp or the mutant variants A-FabpF58S, A-FabpK59E, A-FabpD77G, and A-FabpD78G and incubated with COS-7 lysates (60 μg of total protein) containing murine His-Cgi-58. Bound proteins were detected using anti-His primary and Hrp-conjugated secondary antibody. Plates were developed using tetramethylbenzidine as substrate, and the absorbance was measured at 450/620 nm. b, solid phase assays detecting the binding of wild type A-Fabp or the mutant variants A-FabpD18K, A-FabpK22E, and A-FabpR31E as well as the double mutant A-FabpK22E,R31E to murine His-Cgi-58 expressed in COS-7 cells. Each mutant variant features an exchange in one or two amino acids involved in binding of Hsl to A-Fabp. The mutant variant A-FabpR127Q, which is not capable of binding FAs, was also tested for binding to Cgi-58. Data are shown as mean ± S.D. (n = 4) and are representative of three independent experiments. Statistical difference was determined as compared with A-Fabp control (n.s., nonsignificant; *, p < 0.05; **, p < 0.01; ***, p < 0.001).
