FIGURE 6.
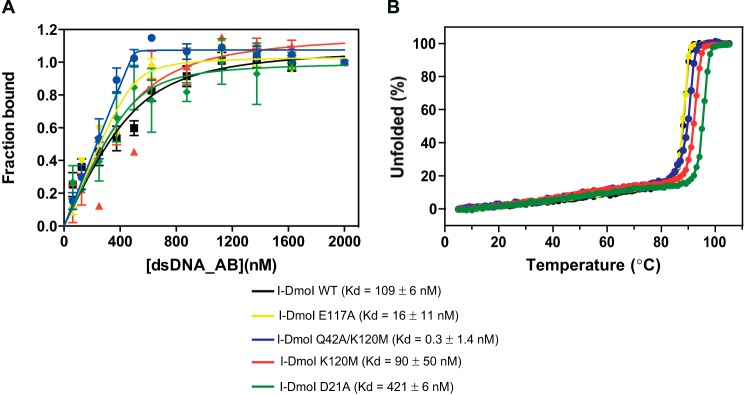
Binding and protein stability of different I-DmoI variants. A, protein-DNA Kd binding of I-DmoI variants. Binding reactions were performed by mixing 500 nm I-DmoI with different concentrations (0–2000 nm) of the 25-bp-long natural I-DmoI target duplex in binding buffer after incubating the mixture for 15 min at 50 °C. Each point was the average of five consecutive measurements. Error bars represent S.D. B, CD thermal denaturation analysis. Thermal denaturation curves were obtained at a protein concentration of 10 μm in a 2-mm cuvette. The ellipticity at 222 nm was recorded at 1 °C/min intervals.
