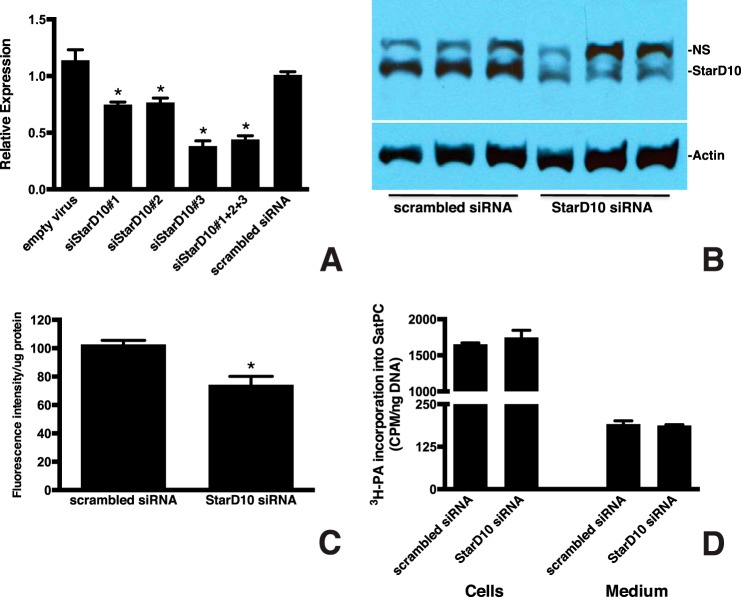
FIGURE 8.
Knockdown of StarD10 results in decreased PC transport to LB. A, qPCR analysis of StarD10 mRNA in mouse type II cells treated with siRNAs targeting StarD10. Freshly isolated mouse type II cells were cultured and transfected with the viral vector alone, scrambled siRNA, and various StarD10 Stealth RNAi siRNAs. The level of endogenous StarD10 mRNA was quantitated by qPCR and normalized to β-actin. Note that StarD10 siRNA 3 was more effective in knocking down StarD10 than siRNA 1, siRNA 2, or a combination of all three. The data represent the mean ± S.E. (error bars) of three independent experiments. *, p < 0.0001 versus scrambled siRNA. B, immunoblot analysis of StarD10 protein levels in whole cell lysates treated with StarD10 siRNA. StarD10 protein was significantly reduced in cells treated with StarD10 siRNA compared with scrambled siRNA. NS, nonspecific band. Results are representative of three independent experiments. C, 72 h after siRNA treatment, type II cells were labeled for 16 h with NBD-palmitoyl-CoA, then LBs were isolated, and the fluorescent content was determined. Incorporation of fluorescent palmitoyl-CoA-labeled phospholipid into LB was normalized to LB protein content. The amount of fluorescent phospholipid that was transported to LB was significantly reduced in cells transfected with StarD10 siRNA compared with scrambled siRNA. The data represent the mean ± S.E. (error bars) of six independent experiments. *, p = 0.0013 versus scrambled siRNA. D, mouse type II cells treated with scrambled or StarD10 siRNA were labeled with [3H]PA, and incorporation into SatPC was determined. [3H]PA incorporation into SatPC was not significantly different in cells treated with scrambled or StarD10 siRNA, indicating that SatPC synthesis itself was unaffected. No change in the amount of SatPC released into the medium was detected as well. The data represent the mean ± S.E. (error bars) of three independent experiments.