FIGURE 1.
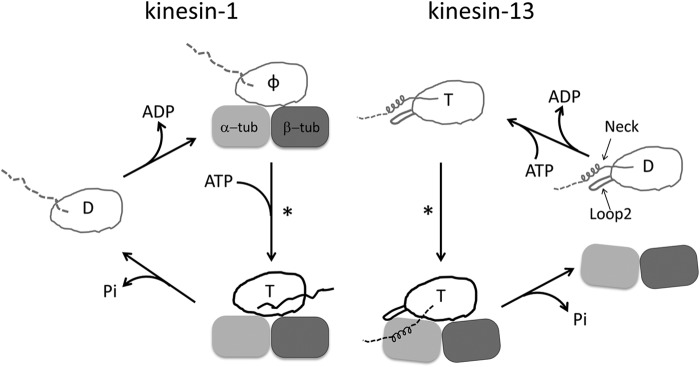
Comparison of the working cycles of motile kinesin-1 and depolymerizing kinesin-13. One motor domain is shown in both cases. The letter inside the motor domain indicates the nucleotide state of kinesin: D, ADP; T, ATP; φ, no nucleotide. In the working cycle of kinesin-1, the microtubule is represented by one tubulin heterodimer. In the ADP binding and nucleotide-free states of kinesin-1, the undocked neck linker is shown as a dashed line, whereas in microtubule-bound kinesin-ATP, the docked neck linker is shown as a solid line pointing toward the microtubule (+) end. In the case of kinesin-13, the subfamily-specific long loop 2 and neck are shown with the motor domain. The microtubule end is shown as a curved tubulin heterodimer. Upon binding to microtubule ends, Kif2C-ATP undergoes a conformational change to form the Kif2C-ATP-tubulin complex, a process to which the neck region contributes significantly (8). However, the binding site of the neck region on tubulin remains unknown, so it is shown in an arbitrary position as a dashed line. In both working cycles, an asterisk indicates the step in which the competent complex forms.
