FIGURE 4.
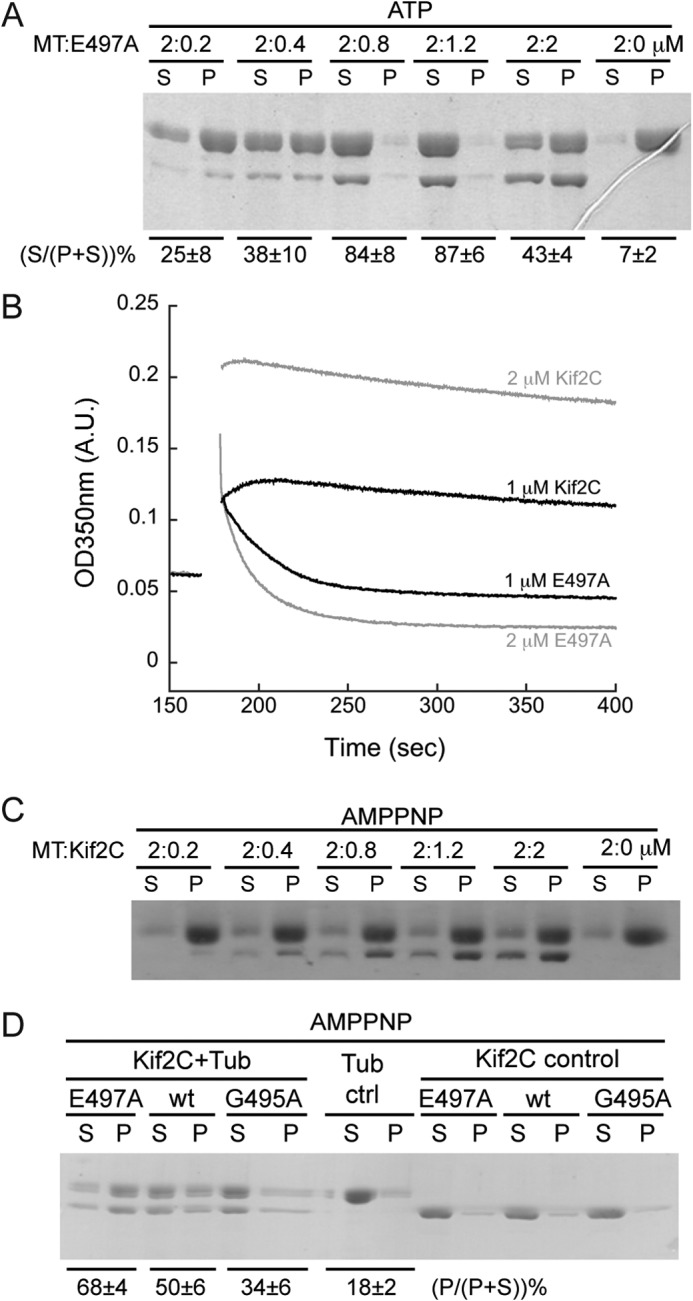
The microtubule depolymerization activity of the ATPase-deficient post-conformational change-mimicking Kif2C-(sN+M)-E497A mutant. A, SDS-PAGE analysis of the supernatant (S) and pellet (P) following ultracentrifugation of 2 μm Taxotere-stabilized microtubules after incubation with various concentrations of Kif2C-(sN+M)-E497A mutant. The relative amount of tubulin band in each lane was quantified with ImageJ, and the percentage of tubulin in the supernatant in each group is given below the gel (average ± S.D., n = 2). B, turbidity traces of 2 μm microtubules depolymerized by 1 μm (black) or 2 μm (gray) Kif2C-(sN+M) or its Kif2C-(sN+M)-E497A mutant in AMPPNP conditions. After adding such high concentrations of Kif2C protein, the turbidity of microtubules had a remarkable, Kif2C concentration-dependent, increase, probably because of the binding of Kif2C onto the microtubule surface. In the case of the E497A mutant, the depolymerization is demonstrated by the quick decrease of turbidity, in clear contrast to the rather stable turbidity in the presence of wild type Kif2C. C, SDS-PAGE analysis of the supernatant (S) and pellet (P) following ultracentrifugation of 2 μm microtubules after incubation with various concentrations of Kif2C-(sN+M) in the presence of 1 mm AMPPNP. D, aggregation of tubulin (0.5 μm) by Kif2C-(sN+M) or its E497A or G495A mutants (0.5 μm) in AMPPNP condition. Following incubation and ultracentrifugation (75,000 rpm), the result was checked by SDS-PAGE. The percentage of tubulin in the pellet in each group is given below the gel (average ± S.D., n = 3).
