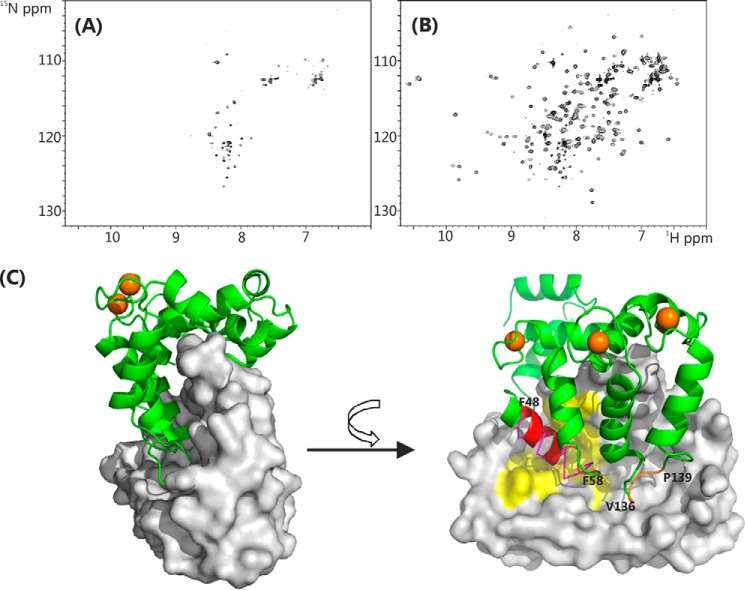
FIGURE 9.
NMR spectra and structure of rat Ca2+/NCS-1ΔCT (PDB code 4YRU). A,1H-15N heteronuclear single quantum coherence spectra of 15N Ca2+/NCS-1ΔCT; B, in the presence of 5-fold excess of D2R peptide in 50 mm Tris-HCl buffer, 50 mm NaCl, 5 mm CaCl2, pH 6.5, 298 K. C, structure of the dimer of NCS-1ΔCT. Left, one monomer is shown as a backbone schematic and the other as a surface representation. Right, view of the hydrophobic binding site. Residues from helix 3 and the EF1/EF2 loop (Phe-48–Phe-58) are colored red, and residues in the EF3/EF4 loop region (Val-136–Pro-139) are colored brown. Conserved hydrophobic surface-exposed residues in the N-lobe site, which are involved with binding the D2R peptide, are highlighted in yellow (similar to those highlighted in Fig. 4B). D2R peptide in the N-site is shown with magenta ribbon for comparison. Ca2+ ions are shown as brown spheres.