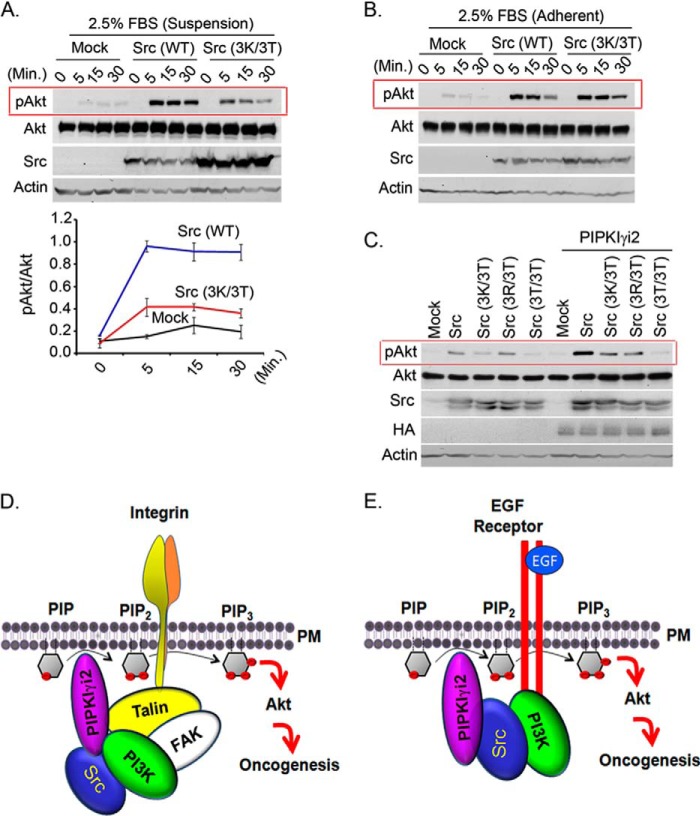
FIGURE 8.
Highly conserved basic amino acids residues in the N terminus of Src are required for Akt activation in synergy with PIPKIγi2. A and B, MDA-MB-231 cells expressing wild type or its mutant form of Src were stimulated in suspension (A) or adherent condition (B) with FBS before examining the activated Akt by immunoblotting. C, NIH3T3 cells co-expressing Src or its mutant form with PIPKIγi2 were cultured in suspension condition for 1–2 days before examining the activated Akt by immunoblotting. D and E, schematic diagram depicting the role of PIPKIγi2 and Src in regulation of PI3K/Akt signaling. PIPKIγi2 is recruited at the vicinity of activated integrins via talin, its direct interacting partner, whereas PI3K can utilize focal adhesion kinase for its recruitment at the adhesion complex upon integrin activation. Simultaneous recruitment of PIP2- and PIP3-synthesizing enzyme may provide the spatial pool of PIP2 and PIP3 required for Akt recruitment to plasma membrane and its subsequent activation, which supports oncogenic growth of tumor cells. Similarly, PIPKIγi2 is recruited in proximity to activated growth factor via Src, which directly interacts with phosphorylated tyrosine motifs of activated growth factor receptors (E). PI3K recognizes distinct tyrosine motifs of activated growth factor receptors. Thus, PIPKIγi2 represents the candidate molecule to contribute the spatial pool of PIP2 required for PIP3 generation and Akt activation upon activation of growth factor receptors and integrins, which promotes oncogenic growth signaling in tumor cells.