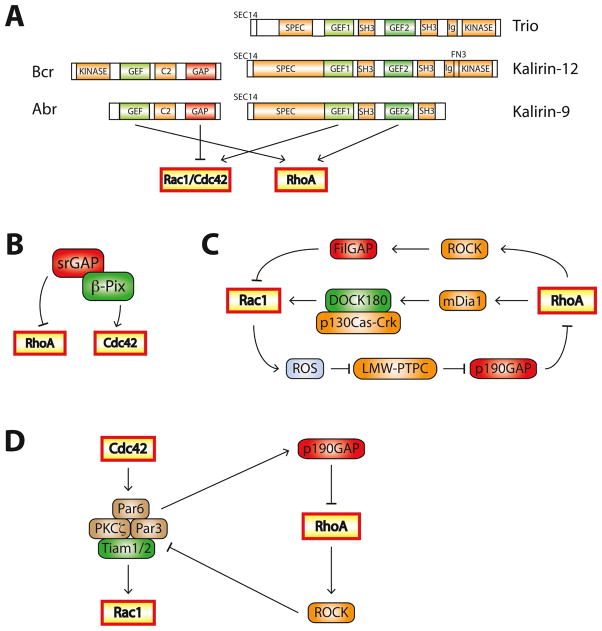
Figure 3. Mechanisms regulating the balance between Rac1/Cdc42 and RhoA.
A. Single proteins can contain multiple Rho-family regulatory domains. Abr and Bcr are both RhoA-GEFs and Rac1/Cdc42-GAPs. Some Rho-family GEFs activate multiple Rho GTPases: Trio, Kalirin-12, and Kalirin-9 are GEFs for RhoA and Rac1/Cdc42. C2: protein kinase C conserved region 2, SEC14: domain in phosphatidylinositol transfer protein Sec14, SPEC: spectrin-like repeats, SH3: Src homology 3 domain, CC: coiled coil, Ig/FN3:Ig/fibronectin III
B. The Cdc42-GEF β-PIX exists in a complex with srGAP, a RhoA-GAP.
C. The Rac1/RhoA balance is tightly regulated through feedback networks. For example, Rac1 inhibits RhoA by inducing ROS production, leading to p190-RhoGAP activation. RhoA increases Rac1 activation through the RhoA effector mDia1, but inhibitsRac1 by activating the Rac1-GAP FilGAP through the RhoA effector ROCK.
D. Crosstalk between Cdc42, Rac1 and RhoA during the establishment of cell polarity. Activated Cdc42 recruits members of the Par complex (Par3, Par6 and PKCζ) to specialized sites to induce cell polarity. Par3 recruits the Rac1-GEFs Tiam1/2, resulting in local Rac1 activation. Par6 interacts with p190-RhoGAP, inhibiting RhoA activity. Conversely, RhoA can disrupt the Par complex through ROCK-dependent phosphorylation of Par3, leading to the Rac1 inhibition.