Figure 2.
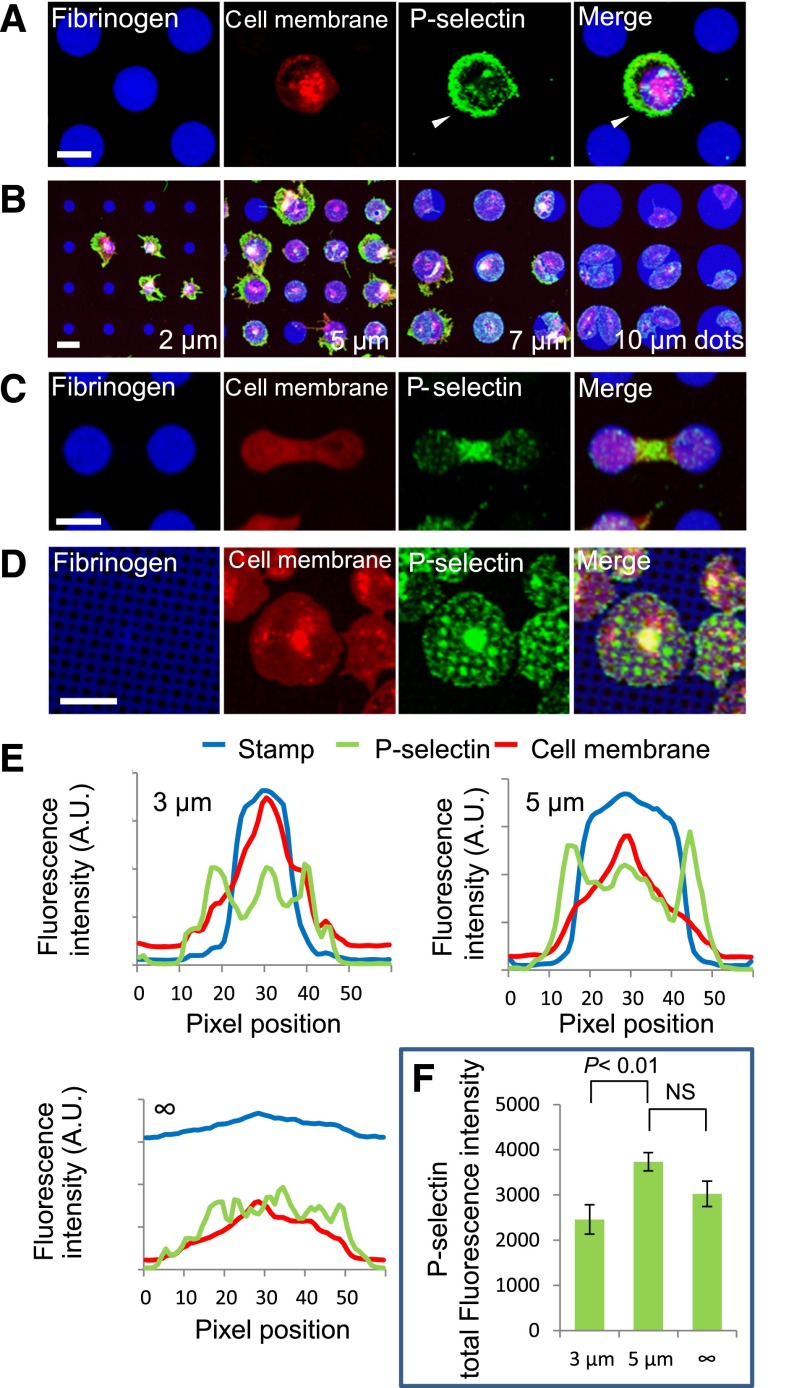
Platelet α-granule secretion is spatially regulated at the micro- and nanoscales. (A) Platelet spreading beyond protein microdot (blue) boundaries is associated with increased P-selectin expression (green) in those specific regions of unpermeabilized platelets (red). Individual fluorescence microscopy channels and a merged image of all 3 channels are shown. (B) High levels of anti–P-selectin staining colocalize with regions of platelets that spread beyond the geometric boundaries of protein microdots, and this effect inversely correlates with microdot size. (C) Permeabilized platelets on adjacent protein microdots form bridging connections associated with high levels of P-selectin in those regions. (D) Protein micropatterns with arrays of nanoscale “holes” reveal that P-selectin colocalizes with the patterned holes in permeabilized platelets. (E) Averaged (n = 7-10 platelets) fluorescence intensity line plots of P-selectin expression (green) on platelets (red, membrane dye) that spread beyond 3- and 5-µm diameter fibrinogen microdots (blue) show peak P-selectin expression outside the micropattern boundaries, whereas no clear peak is shown in platelets that spread on evenly coated fibrinogen surfaces. (F) Total P-selectin signal intensity is significantly increased on platelets spread on 5-µm diameter microdots compared with platelets on 3-μm microdots, but was not significantly different compared with platelets on evenly coated surfaces (“∞” µm diameter). The scale bars = 5 µm.