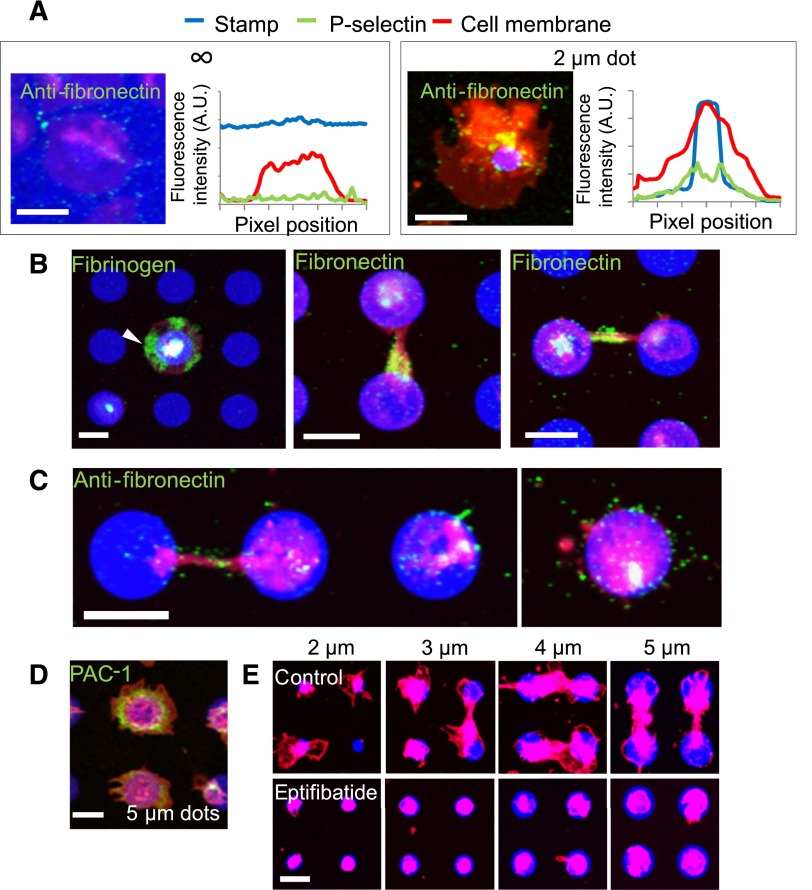
Figure 4.
Matrix self-deposition by spatially regulated α-granule secretion supports platelet spreading beyond the geometric boundaries of the protein micropattern in an αIIbβ3-dependent manner. (A) Immunostaining with antifibronectin reveals that small amounts of fibronectin (green) colocalized with the platelet membrane (red) margins on platelets spread on evenly coated fibrinogen (blue) surfaces (left) but shows increased levels of fibronectin beyond the protein micropattern boundaries (right). Averaged (n = 5-8 platelets) fluorescence intensity line plots of fibronectin expression show that the fibronectin signal is highest at and immediately beyond the micropattern boundaries and not at the edge of platelet membrane. (B) Regions of platelets (red) that spread beyond the collagen microdot (blue) boundaries stain strongly with antifibrinogen (left, green, arrow). Strong expression of fibronectin was also observed at connections between 2 platelets adhered to adjacent protein microdots (middle), and also at the area around the connection (right). (C) Retraction of spread platelets via latrunculin A exposure reveal the deposited fibronectin (green) that remains on the surface after retraction of the platelet membrane. (D). Live cell staining with FITC-PAC-1 (green) shows high concentrations of activated αIIbβ3 integrins distributed at and beyond the geometric boundaries of fibrinogen microdots (blue). (E) When platelets (red) were treated with eptifibatide, an αIIbβ3 antagonist, platelet spreading beyond the geometric constraints of the collagen micropattern (blue) is completely inhibited. Platelets (B,E) were permeabilized upon staining. The scale bars= 5 µm.