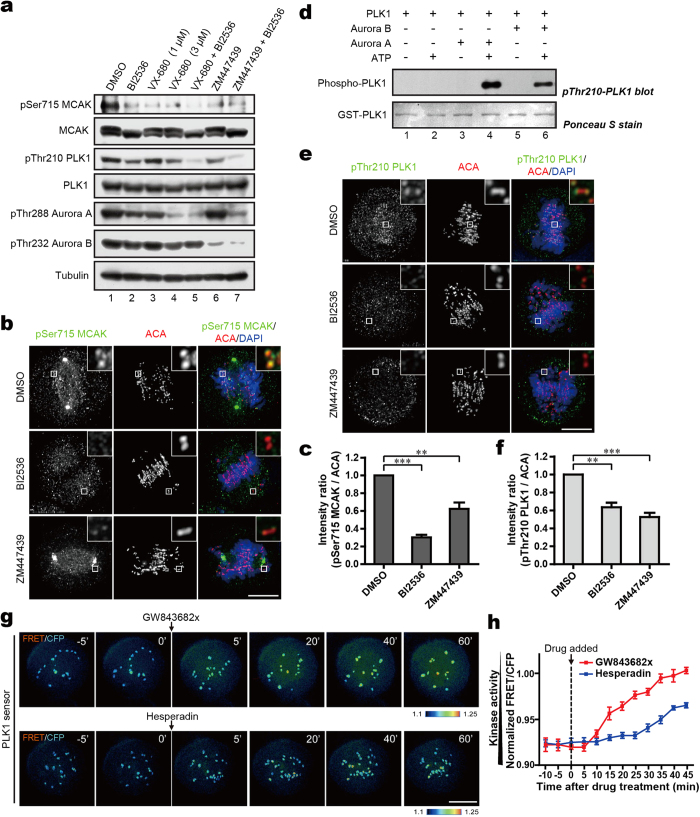
Figure 5. Aurora B is involved in PLK1-dependent phosphorylation of MCAK through activation of PLK1 at the centromeres.
(a) HeLa cells synchronized to mitosis by Nocodazole were treated respectively with BI2536, VX-680, ZM447439 or DMSO for another 1 hr, followed by Western blot analysis with the indicated antibodies (b,e) Synchronized mitotic HeLa cells were treated with BI2536, ZM447439 or DMSO for 1 hr. Cells were then fixed and stained with anti-pSer715-MCAK antibody or anti-pThr210-PLK1 antibody (green), ACA (red) and DAPI (blue). The enlargements show the representative centromeres. (c,f). Statistical analysis of pSer715-MCAK and pThr210-PLK1 immunofluorescence intensity at centromeres in (b) and (e), respectively. The intensity ratio in DMSO-treated group was normalized to 1. Data are shown as means ± SE and derived from at least 10 cells for each condition. **P < 0.01, ***P < 0.001, Student’s t-test. (d) Recombinant GST-PLK1 was phosphorylated by Aurora kinases. In vitro phosphorylation reactions were performed as described in Methods. GST-PLK1 was visualized by Ponceau S staining and phosphorylation was detected by the antibody against pThr210-PLK1 by Western blotting. (g) Color-coded images of HeLa cells expressing a centromere-targeted PLK1 FRET sensor in prometaphase of cells arrested by Taxol treatment. PLK1 inhibitor (GW843682x) or Aurora B inhibitor (Hesperadin) was added at the indicated time point during live-cell imaging. Timestamps relative to drug addition is annotated in min. (h) Statistical analysis of the FRET/CFP emission ratio on centromeres at the indicated time (g). Data are presented as means ± SE derived from over 100 kinetochores of each category from five different cells. Scale bars, 10 μm (all image panels).