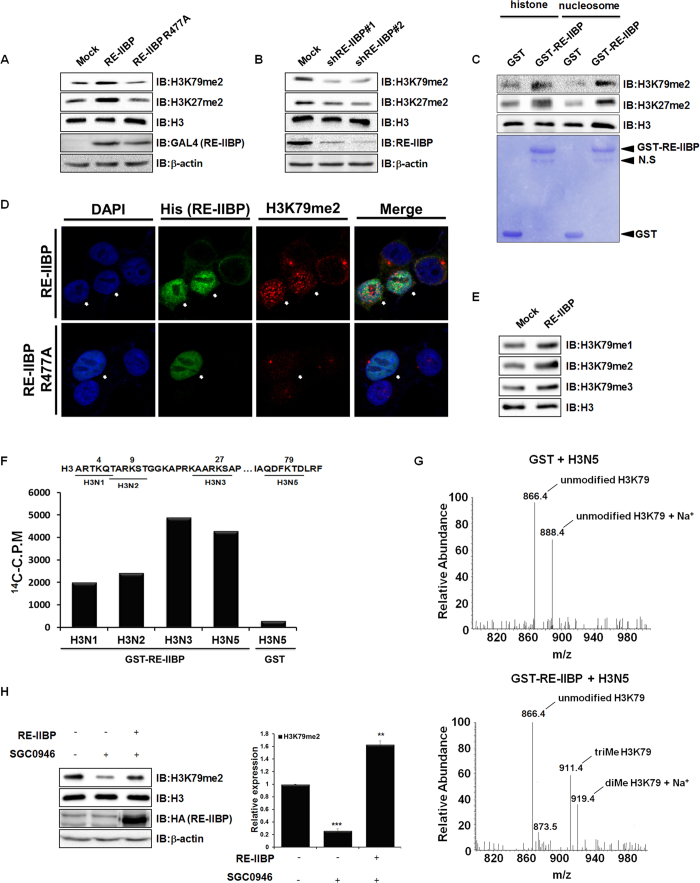
Figure 1. Histone H3K79 HMTase Activity of RE-IIBP.
(a) and (b), H3K79me2 and H3K27me2 levels were analyzed in RE-IIBP or RE-IIBP R477A transfected 293T cells and stably knocked-down 293T cells using two independent shRNAs. Purified histones were immunoblotted with anti-H3K79me2, anti-H3K27me2, anti-RE-IIBP and anti-GAL4 antibodies. β-actin and H3 were used as loading controls. (c), Core histones and nucleosomes were used as substrates in the in virto HMTase assay with GST and GST-RE-IIBP. Incubated core histones and nucleosomes were subjected to Western blot. The amount of GST-RE-IIBP was determined by Coomassie staining. (d), 293T cells transiently transfected with His-RE-IIBP or His-RE-IIBP R477A were fixed, permeabilized, and immunostained with anti-His and anti-H3K79me2 antibodies. Nuclei were counterstained with DAPI. (e), Immunoblot analyses show the relative methylation levels of H3K79 in RE-IIBP transfected 293T cells. Purified histones using histone purification assay were immunoblotted with anti-H3K79me1, anti-H3K79me2, and anti-H3K79me3 antibodies. The methylation levels are normalized to H3. (f), HMTase assays were performed with four synthesized peptides, shown above, as substrates. Peptide methylations were measured using a scintillation counter. (g), Synthesized peptides (H3N5) were used as substrates in the HMTase assay with purified GST or GST-RE-IIBP. After 3 h, the peptides were analyzed by LC-MS. (h), SGC0946, DOT1L inhibitor, mediated H3K79me2 levels were restored by RE-IIBP. 293T cells treated with 1 nM SGC0946 were transiently transfected with RE-IIBP. Cells were lysed or used for histone purification assay. Each extracts were immunoblotted with anti-H3K79me2, anti-H3, anti-HA and anti-β-actin antibodies (left). The relative expression levels of H3K79me2 were quantified (right). Results are shown as means ± SDs n = 3; **p < 0.01, ***p < 0.001