Abstract
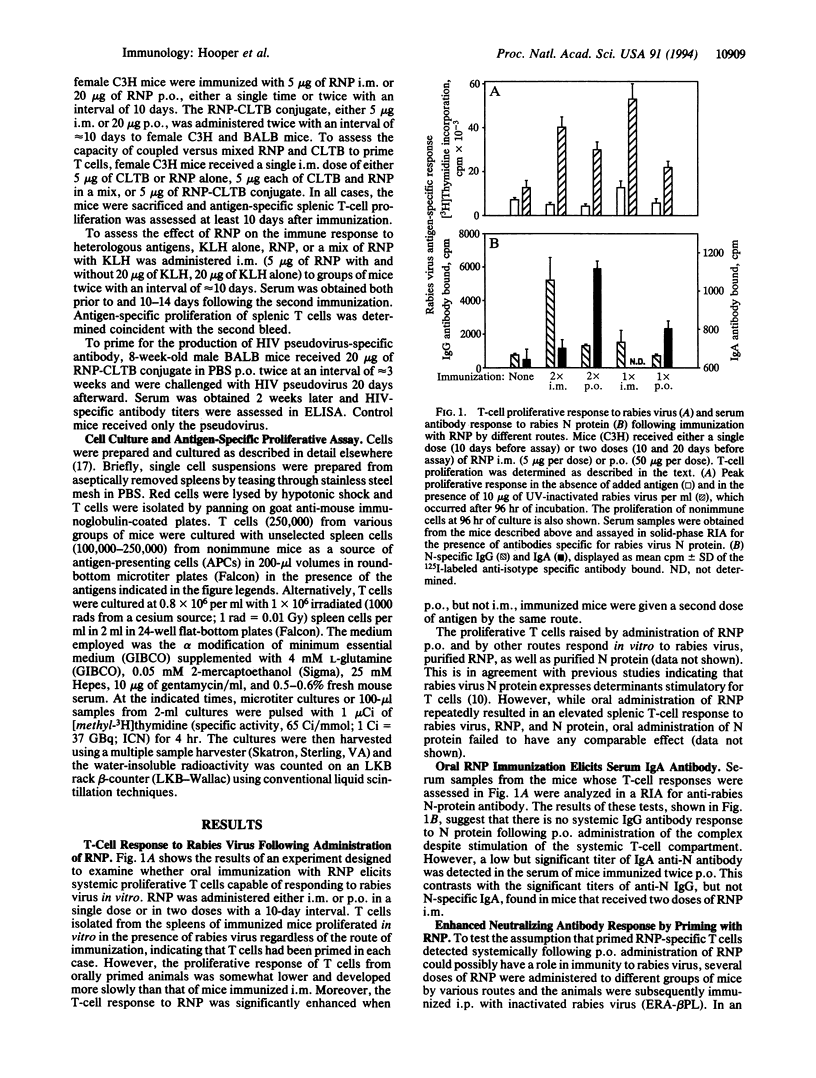
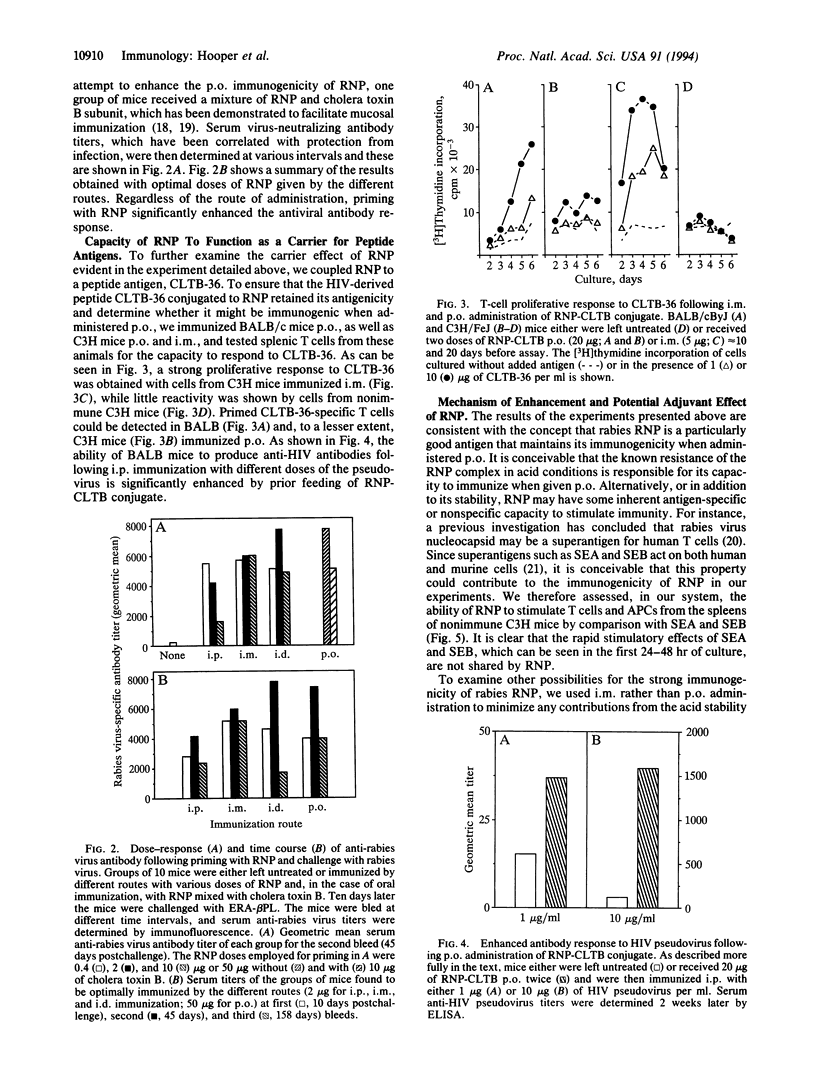
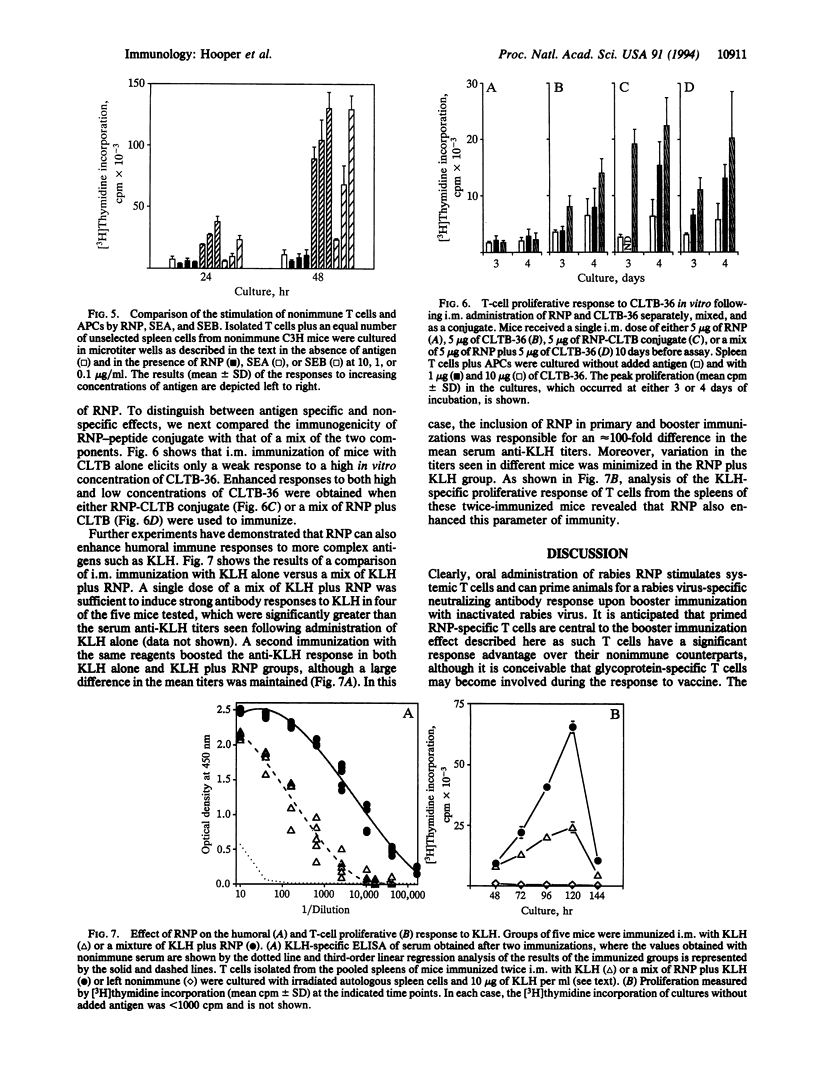
The administration of rabies ribonucleocapsid (RNP) by oral as well as parenteral routes was found to prime specific T cells and elicit N-protein-specific antibodies. per os and intramuscular immunization led to the production of antibodies of the IgA and IgG isotypes, respectively. Mice primed orally with RNP produced significantly enhanced amounts of virus-neutralizing antibody, compared with non-immune controls, upon subsequent parenteral booster immunization with inactivated rabies virus. Thus oral immunization with rabies RNP primed cells capable of mediating a secondary systemic response to rabies virus. The results of experiments in which peptide and protein antigens were administered either physically coupled to or mixed with RNP indicate that RNP has an inherent capacity to enhance immune responses.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brochier B., Kieny M. P., Costy F., Coppens P., Bauduin B., Lecocq J. P., Languet B., Chappuis G., Desmettre P., Afiademanyo K. Large-scale eradication of rabies using recombinant vaccinia-rabies vaccine. Nature. 1991 Dec 19;354(6354):520–522. doi: 10.1038/354520a0. [DOI] [PubMed] [Google Scholar]
- Burtles S. S., Taylor R. B., Hooper D. C. Bovine gamma globulin-specific CD4+ T cells are retained by bovine gamma-globulin-tolerant mice. Eur J Immunol. 1990 Jun;20(6):1273–1279. doi: 10.1002/eji.1830200612. [DOI] [PubMed] [Google Scholar]
- Dietzschold B., Wang H. H., Rupprecht C. E., Celis E., Tollis M., Ertl H., Heber-Katz E., Koprowski H. Induction of protective immunity against rabies by immunization with rabies virus ribonucleoprotein. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9165–9169. doi: 10.1073/pnas.84.24.9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertl H. C., Dietzschold B., Gore M., Otvos L., Jr, Larson J. K., Wunner W. H., Koprowski H. Induction of rabies virus-specific T-helper cells by synthetic peptides that carry dominant T-helper cell epitopes of the viral ribonucleoprotein. J Virol. 1989 Jul;63(7):2885–2892. doi: 10.1128/jvi.63.7.2885-2892.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z. F., Dietzschold B., Schumacher C. L., Wunner W. H., Ertl H. C., Koprowski H. Rabies virus nucleoprotein expressed in and purified from insect cells is efficacious as a vaccine. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):2001–2005. doi: 10.1073/pnas.88.5.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z. F., Rupprecht C. E., Dietzschold B., Saikumar P., Niu H. S., Babka I., Wunner W. H., Koprowski H. Oral vaccination of racoons (Procyon lotor) with baculovirus-expressed rabies virus glycoprotein. Vaccine. 1993;11(9):925–928. doi: 10.1016/0264-410x(93)90379-c. [DOI] [PubMed] [Google Scholar]
- Hoyne G. F., Callow M. G., Kuhlman J., Thomas W. R. T-cell lymphokine response to orally administered proteins during priming and unresponsiveness. Immunology. 1993 Apr;78(4):534–540. [PMC free article] [PubMed] [Google Scholar]
- Labrecque N., Thibodeau J., Sékaly R. P. T-cell receptor recognition of superantigens: another view. Res Immunol. 1993 Mar-Apr;144(3):175–180. doi: 10.1016/0923-2494(93)80113-d. [DOI] [PubMed] [Google Scholar]
- Lafon M., Lafage M., Martinez-Arends A., Ramirez R., Vuillier F., Charron D., Lotteau V., Scott-Algara D. Evidence for a viral superantigen in humans. Nature. 1992 Aug 6;358(6386):507–510. doi: 10.1038/358507a0. [DOI] [PubMed] [Google Scholar]
- Liang X. P., Lamm M. E., Nedrud J. G. Oral administration of cholera toxin-Sendai virus conjugate potentiates gut and respiratory immunity against Sendai virus. J Immunol. 1988 Sep 1;141(5):1495–1501. [PubMed] [Google Scholar]
- Liu F. T., Zinnecker M., Hamaoka T., Katz D. H. New procedures for preparation and isolation of conjugates of proteins and a synthetic copolymer of D-amino acids and immunochemical characterization of such conjugates. Biochemistry. 1979 Feb 20;18(4):690–693. doi: 10.1021/bi00571a022. [DOI] [PubMed] [Google Scholar]
- Melamed D., Friedman A. Direct evidence for anergy in T lymphocytes tolerized by oral administration of ovalbumin. Eur J Immunol. 1993 Apr;23(4):935–942. doi: 10.1002/eji.1830230426. [DOI] [PubMed] [Google Scholar]
- Rupprecht C. E., Dietzschold B., Campbell J. B., Charlton K. M., Koprowski H. Consideration of inactivated rabies vaccines as oral immunogens of wild carnivores. J Wildl Dis. 1992 Oct;28(4):629–635. doi: 10.7589/0090-3558-28.4.629. [DOI] [PubMed] [Google Scholar]
- Rupprecht C. E., Wiktor T. J., Johnston D. H., Hamir A. N., Dietzschold B., Wunner W. H., Glickman L. T., Koprowski H. Oral immunization and protection of raccoons (Procyon lotor) with a vaccinia-rabies glycoprotein recombinant virus vaccine. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7947–7950. doi: 10.1073/pnas.83.20.7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider L. G., Dietzschold B., Dierks R. E., Matthaeus W., Enzmann P. J., Strohmaier K. Rabies group-specific ribonucleoprotein antigen and a test system for grouping and typing of rhabdoviruses. J Virol. 1973 May;11(5):748–755. doi: 10.1128/jvi.11.5.748-755.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H. C., Parrott M. V. The induction of tolerance to a soluble protein antigen by oral administration. Immunology. 1974 Oct;27(4):631–639. [PMC free article] [PubMed] [Google Scholar]
- Tomasi T. B., Jr Oral tolerance. Transplantation. 1980 May;29(5):353–356. doi: 10.1097/00007890-198005000-00001. [DOI] [PubMed] [Google Scholar]
- Vajdy M., Lycke N. Y. Cholera toxin adjuvant promotes long-term immunological memory in the gut mucosa to unrelated immunogens after oral immunization. Immunology. 1992 Mar;75(3):488–492. [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., Dietzschold B., Leamnson R. N., Koprowski H. Induction and biological properties of defective interfering particles of rabies virus. J Virol. 1977 Feb;21(2):626–635. doi: 10.1128/jvi.21.2.626-635.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., Macfarlan R. I., Foggin C. M., Koprowski H. Antigenic analysis of rabies and Mokola virus from Zimbabwe using monoclonal antibodies. Dev Biol Stand. 1984;57:199–211. [PubMed] [Google Scholar]


