Abstract
An elderly woman with a history of pulmonary tuberculosis reportedly diagnosed and treated 30 years prior to presentation was found unresponsive at home. Chest imaging revealed innumerable pulmonary nodules worrisome for an infectious process, specifically tuberculosis. The patient deteriorated rapidly and in accordance with her wishes, aggressive interventions were withheld. She died within 48 h from respiratory failure. A limited chest autopsy was performed and revealed the cause of death as lymphangitic spread of cancer from a primary lung adenocarcinoma.
Background
Miliary shadowing is a classic pattern on chest radiography, referring to the collection of numerous micronodules interspersed within the lung parenchyma. While classically associated with disseminated tuberculosis, miliary shadowing may be the manifestation of a host of other conditions, including interstitial lung disease, infections and malignancy. Through careful interpretation of the radiographs, in conjunction with the history, physical examination and laboratory studies, it may be possible to significantly narrow the differential diagnosis. We illustrate this in the case of an 88-year-old woman with prior tuberculosis who presented with acute respiratory failure, and further review the approach to interpreting a miliary pattern on chest imaging.
Case presentation
An 88-year-old woman with a history of Graves’ disease and hypertension presented to the emergency department after being found slumped in a chair at home. She was last seen well approximately 4 h before the incident. Her family relayed that she had developed increasing shortness of breath and a dry, non-productive cough over the past 2 months associated with decreased appetite and a loss of approximately 4.5 kg. The family also denied the patient having fevers or night sweats. The patient emigrated from Vietnam to USA 12 years prior. While in Vietnam, she had been diagnosed with pulmonary tuberculosis approximately 30 years earlier, and was treated for 9 months with rifampin, isoniazid, pyrazinamide and ethambutol. Her family stated that she was a heavy smoker with a 60 pack year history, but that she had quit over 45 years ago.
On initial presentation, the patient's vital signs were within normal limits. She was arousable and responded to simple commands. Chest examination revealed vesicular breath sounds bilaterally without rales, rhonchi or wheezes. There was no deviation of the trachea, and no cervical lymphadenopathy, goitre, clubbing of nails or temporal wasting.
Investigations
Initial laboratory evaluation, including serum electrolyte panel, liver function panel and complete blood count, was significant for hyponatraemia (127 mEq/L) and leucocytosis (13.9 k/µL). Thyroid stimulating hormone was within normal limits.
A supine anteroposterior chest X-ray was performed, showing a miliary pattern bilaterally with a possible small left-sided pleural effusion (figure 1). This was followed by CT of the chest without contrast, which showed innumerable perilymphatic pulmonary nodules that formed conglomerate areas of consolidation in lower lung zones, and confirmed the small pleural effusion (figure 2). The patient was placed on respiratory isolation. Blood and sputum cultures, including those for fungi and acid-fast bacilli, were negative.
Figure 1.

Supine anteroposterior chest X-ray.
Figure 2.
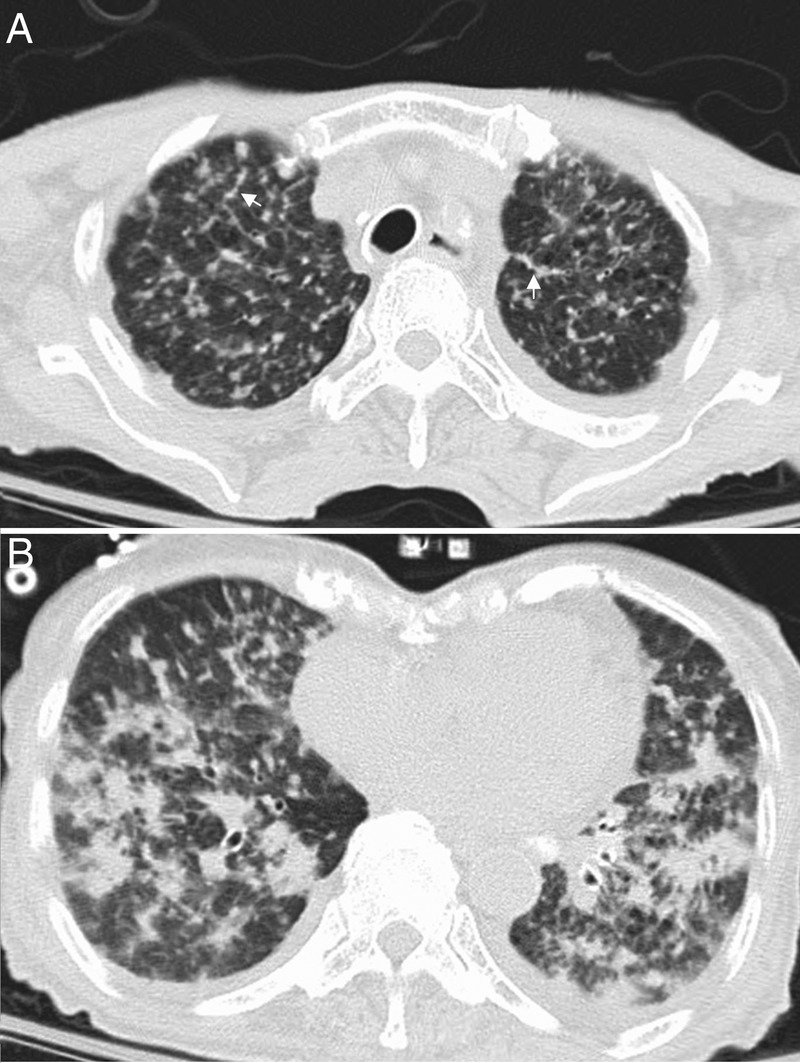
Non-contrasted chest CT of the (A) upper lobes showing miliary nodules with interlobular septal thickening (arrows) and (B) lower lobes showing diffuse conglomerate areas of consolidation.
Outcome and follow-up
By 36 h, the patient's mental and respiratory status had worsened. The patient's family decided not to pursue further work up, in line with the patient's known wishes, and plans for bronchoscopy to obtain biopsy samples were cancelled. The patient died within 6 h. The family granted an autopsy, which was performed 12 h postmortem.
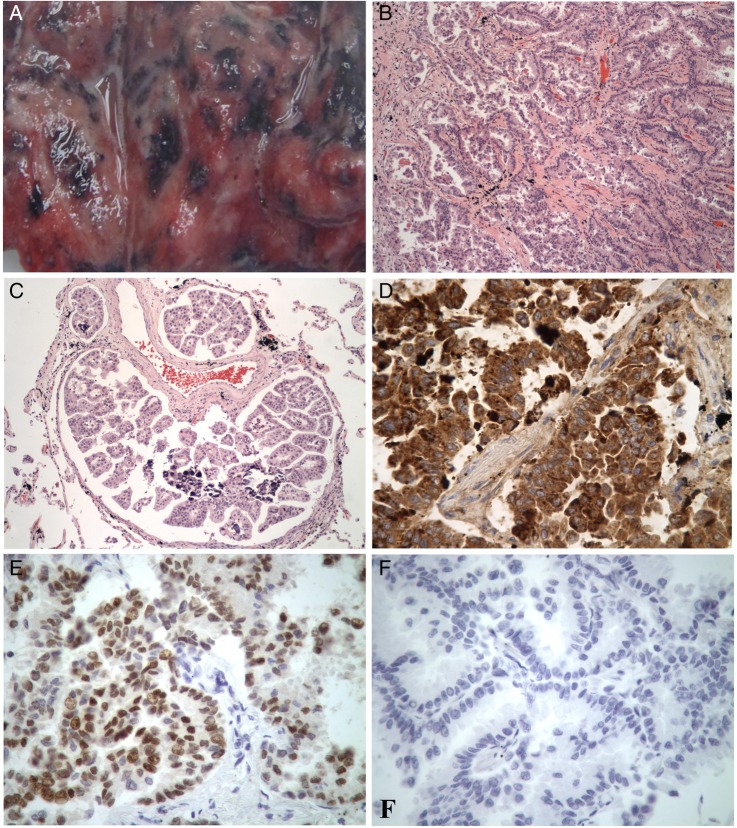
With significant clinical suspicion for recurrence of pulmonary tuberculosis, the autopsy was limited to the chest. Wedge biopsies of the left superior and left inferior pulmonary lobes were obtained, revealing diffuse anthracosis with partially confluent, variably sized white nodules along the entirety of the visceral pleural and parenchymal surfaces (figure 3A). The parietal pleura was uninvolved on gross inspection and palpation.
Figure 3.
(A) Gross image of lung biopsy. (B) Histological section of lung tissue depicting diffuse adenocarcinoma, ×40 (stain; H&E). (C) Histological section depicting diffuse lymphangitic involvement, ×40 (H&E). (D) Napsin A staining of lung tissue, ×400. (E) TTF-1 staining of lung tissue, ×400. (F) Thyroglobulin staining of lung tissue, ×400. TTF-1, thyroid transcription factor 1.
Representative histological sections revealed extensive infiltrating adenocarcinoma with moderate nuclear pleomorphism and dense eosinophilic cytoplasm on light microscopy (figure 3B). Glands were characterised by a complex branching papillary configuration. Extensive lymphangitic spread was noted in all sections (figure 3C).
Immunohistochemical analysis was required to characterise the aetiology of the carcinoma. Staining was positive for napsin A and thyroid transcription factor 1 (TTF-1) and negative for thyroglobulin (figure 3D–F). Lung tissue cultures and postmortem blood cultures were negative for bacterial, mycobacterial or fungal isolates.
Discussion
The patient's antecedent 2 month history of cough, weight loss and anorexia strongly suggested that this event was the culmination of an underlying chronic process. Certainly, the patient's history of active pulmonary tuberculosis and miliary pattern on chest X-ray led to concerns that the patient may have had reactivation of tuberculosis. The incidence of pulmonary tuberculosis among US emigrants represents 63% of all new cases in this country and most commonly reflects reactivation of latent tuberculosis.1 However, reactivation of latent infection occurs at a rate fivefold higher within the first 5 years of emigration relative to succeeding years, and the incidence of reactivation in HIV-negative patients is relatively uncommon, with an estimated frequency of 4.3 cases per 100 patient-years at risk.1 2 Other possible infectious aetiologies included fungal, viral and other atypical pneumonias. Alternatively, the patient's advanced age and extensive smoking history suggested that she may have had a primary lung malignancy. Prolific metastatic disease was also considered, with the patient's long-standing history of Graves’ disease placing her at an elevated risk for thyroid malignancy.3
Though lung adenocarcinoma is overall the most common subtype of non-small cell lung cancer in Western countries, diagnosis is often difficult to establish since patients most commonly present with non-specific symptoms, including fatigue, dyspnoea and cough. It is also common for patients to present after metastasis, although lung adenocarcinoma tends to spread to distant sites such as the liver, bone, brain and adrenal glands, rather than to the pulmonary lymphatic system.4 5
In the absence of definitive microbiological or histological data, the most discriminant feature of this patient's presentation was the miliary pattern found on chest imaging. Named after its resemblance to millet seeds, miliary shadowing is a classical pattern, first recognised in chest radiographs, referring to the presence of numerous small nodules. Though non-specific, miliary shadowing represents diffuse micronodular lung disease, and is associated with such illnesses as miliary tuberculosis, hypersensitivity pneumonia, lung infections, sarcoidosis, pneumoconiosis and metastatic cancers (table 1). A far less common cause of miliary shadowing is primary adenocarcinoma of the lung.6
Table 1.
Common aetiologies of miliary shadowing on high-resolution CT (HRCT) scans
| Aetiology | Distribution | Associated findings | Comments |
|---|---|---|---|
| Hypersensitivity pneumonitis | Centrilobular distribution of nodules (5 mm nodules) | Subacute: ground-glass opacities Chronic: interstitial fibrosis, often with loss of micronodules |
Nodules correspond to poorly circumscribed granulomas and acute alveolitis around central area of lobule |
| Viral pneumonia | Centrilobular distribution with branching structures | Cavitated nodules and coalescent lesions | Sharply defined nodules, often called ‘pseudo-miliary’ due to patchy distribution |
| Sarcoidosis | Irregular contoured nodules in perilymphatic distribution | Ground-glass attenuation and thickening of bronchovascular bundles, enlarged lymph nodes | In less than 1% of cases, may be a random distribution |
| Pneumoconiosis (ie, silicosis and coal worker's pneumoconiosis) | Perilymphatic and/or random distribution with upper lobe predominance. Commonly found subpleurally | Hilar and mediastinal enlargement and calcification in “eggshell pattern” | Nodules are typically 2–5 mm in diameter |
| Miliary tuberculosis | Multiple, well-defined nodules in a random pattern | Ground-glass areas with variable extension and patchy appearance. Pleural effusions and mediastinal lymphadenopathy | Most are 1–3 mm and usually less than 5 mm. Larger macronodular opacities may be due to convergence of multiple granulomas or airspace nodules |
| Metastatic cancer with haematogenous spread | Random distribution of large micronodules (usually >5 mm) | An isolated macronodule may correspond to a primary tumour | Associated with vascular primary tumours (eg, renal cell carcinoma, breast carcinoma, melanoma). In thyroid carcinoma, may be first sign of malignancy |
| Lymphangitic carcinomatosis | Perilymphatic distribution, usually in the lower lobes. Linear or reticular pattern early in disease | Thickening of bronchovascular bundles. Pleural effusions and lymphadenopathy | Breast cancer is the most common cause, followed by stomach cancer and pancreatic cancer |
The increased use of high-resolution CT (HRCT) has helped to define these lesions further. The distribution and characteristics associated with these micronodules can help to narrow the differential diagnosis further. In general, three types of distribution have been characterised: centrilobular, perilymphatic and random (table 1).4 5
Centrilobular lesions are located far from the pleurae and often occur in branching linear structures. On chest X-rays, the micronodules are often ill-defined and so are termed ‘pseudo-miliary,’ although differentiation between the two makes little difference in practice.4 This pattern implies pathology of the bronchioles and adjacent peribronchiolar airspaces, such as in bronchiolitis, hypersensitivity pneumonias, bronchopulmonary carcinoma and infections, including endotracheal tuberculosis. Features such as ground-glass opacities and branching structures can further help in narrowing the differential diagnosis.7
In contrast, perilymphatic nodules characteristic of lymphatic spread in sarcoidosis and silicosis are often seen in the subpleural space, along fissures or interlobular septae, and are classically distributed in the upper lobes.5 Lymphangitic carcinomatosis manifests similarly, but is characterised by basilar prominence and nodularity of interlobular septae, often concomitantly associated with effusions and discrete lung nodules. Among cancers that produce such a pattern, breast, lung, stomach and colon cancers are the most common.8
Finally, micronodules can also appear in a random distribution with numerous variably sized nodules, suggesting haematogenous spread, as seen in tuberculosis and metastatic cancers. The number and size of nodules on HRCT has not been correlated with the clinical course in tuberculosis, but this is typically a late feature of the disease and is associated with poor outcomes.9 In contrast, miliary distribution of metastases may occur earlier in certain cancers, such as thyroid cancer.10
Despite being the most worrisome potential diagnosis, the radiographic evidence for miliary tuberculosis in our patient was rather weak. The distribution and size of the nodules along with preferential involvement of the lower lobes was only consistent with lymphangitic carcinomatosis.11
Pulmonary lymphangitic carcinomatosis is a rare manifestation of metastatic disease characterised by severe progressive dyspnoea. The suggested mechanism of spread is retrograde permeation of the pulmonary lymphatics after involvement of hilar lymph nodes. Obliterative changes in pulmonary arteries are common and may suggest haematogenous spread with subsequent perivascular lymphatic permeation.12
Although almost any metastatic cancer can produce this pattern, primary tumours originating from the breast (33%), stomach (29%) and lung (17%), are most frequently encountered.13 Primary bronchopulmonary carcinomas tend to arise either from the parenchyma or bronchus and metastasise to hilar nodes as a nidus for bilateral lymphatic spread.14
The histological pattern in the present case was characterised by extensively infiltrating papillary adenocarcinoma. Complex lymphovascular branching was interrupted by a dense desmoplastic reaction resulting in lymphangiectasia, resulting in the pleural effusion. Because papillary configuration of glands can also be seen in papillary thyroid carcinoma and is known to produce a miliary pattern on chest radiography, immunohistochemical staining was required to distinguish between the two.15 Ultimately, the cells stained positive for napsin A, which has a 87–94% sensitivity for lung adenocarcinoma, and were negative for thyroglobulin, which carries a 95% sensitivity and specificity for papillary thyroid carcinoma.16 17 TTF-1 is highly expressed in lung adenocarcinomas and small cell carcinomas (75–90% sensitivity) with positive staining ruling out metastatic adenocarcinomas of the breast, colon, kidney and stomach.18
Learning points.
Miliary shadowing on chest radiography has a broad differential diagnosis, and should be interpreted critically and in conjunction with relevant clinical and laboratory data.
There are three patterns of involvement on CT of the chest—centrilobular, perilymphatic and random. Distribution can help narrow down differential diagnosis to only a few possibilities.
Lymphangitic carcinomatosis presents with miliary shadowing in a perilymphatic pattern with predominant lower lobe involvement, and is most commonly associated with breast cancer.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Glynn JR, Murray J, Bester A et al. High rates of recurrence in HIV-infected and HIV-uninfected patients with tuberculosis. J Infect Dis 2010;201:704–11. 10.1086/650529 [DOI] [PubMed] [Google Scholar]
- 2.Antonelli A, Ferrari SM, Corrado A et al. Autoimmune thyroid disorders. Autoimmun Rev 2014;14:174–80. 10.1016/j.autrev.2014.10.016 [DOI] [PubMed] [Google Scholar]
- 3.Walter ND, Painter J, Parker M et al. Tuberculosis Epidemiologic Studies Consortium. Persistent latent tuberculosis reactivation risk in United States immigrants. Am J Respir Crit Care Med 2014;189:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaikwad A, Gupta A, Hare S et al. Primary adenocarcinoma of lung: a pictorial review of recent updates. Eur J Radiol. 2012;81:4146–55. 10.1016/j.ejrad.2012.08.023 [DOI] [PubMed] [Google Scholar]
- 5.McGuinness G, Naidich DP, Jagirdar J et al. High resolution CT findings in miliary lung disease. J Comput Assist Tomogr 1992;16:384–90. 10.1097/00004728-199205000-00009 [DOI] [PubMed] [Google Scholar]
- 6.Andreu J, Mauleón S, Pallisa E et al. Miliary lung disease revisited. Curr Probl Diagn Radiol 2002;31:189–97. 10.1067/mdr.2002.127634 [DOI] [PubMed] [Google Scholar]
- 7.Abbott GF, Rosado-de-Christenson ML, Rossi SE et al. Imaging of small airways disease. J Thorac Imaging 2009;24:285–98. 10.1097/RTI.0b013e3181c1ab83 [DOI] [PubMed] [Google Scholar]
- 8.Collins J. CT signs and patterns of lung disease. Radiol Clin North Am 2001;39:1115–35. 10.1016/S0033-8389(05)70334-1 [DOI] [PubMed] [Google Scholar]
- 9.Kwong JS, Carignan S, Kang E-Y et al. Miliary tuberculosis. Diagnostic accuracy of chest radiography. Chest 1996;110:339–42. 10.1378/chest.110.2.339 [DOI] [PubMed] [Google Scholar]
- 10.Filderman AE, Coppage I, Shaw C et al. Pulmonary and pleural manifestation of extrathoracic malignancies. Clin Chest Med 1989;10:747–807. [PubMed] [Google Scholar]
- 11.Thomas A, Lenox R. Pulmonary lymphangitic carcinomatosis as a primary manifestation of colon cancer in a young adult. CMAJ 2008;179:338–40. 10.1503/cmaj.080142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandler GN, Telling M. Lymphangitis carcinomatosa. Br Med J 1952;2:639–41. 10.1136/bmj.2.4785.639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruce DM, Heys SD, Eremin O. Lymphangitis carcinomatosa: a literature review. J R Coll Surg Edinb 1996;41:7–13. [PubMed] [Google Scholar]
- 14.Yang SP, Lin CC. Lymphangitic carcinomatosis of the lungs. The clinical significance of its roentgenologic classification. Chest 1972;62:179–87. 10.1378/chest.62.2.179 [DOI] [PubMed] [Google Scholar]
- 15.Fend F, Gruber U, Fritzsche H et al. Occult papillary carcinoma of the thyroid with pulmonary lymphangitic spread diagnosed by lung biopsy. Klin Wochenschr 1989;67:687–90. 10.1007/BF01718031 [DOI] [PubMed] [Google Scholar]
- 16.Gremel G, Bergman J, Djureinovic D et al. A systematic analysis of commonly used antibodies in cancer diagnostics. Histopathology 2014;64:293–305. 10.1111/his.12255 [DOI] [PubMed] [Google Scholar]
- 17.Turner BM, Cagle PT, Sainz IM et al. Napsin A, a new marker for lung adenocarcinoma, is complementary and more sensitive and specific than thyroid transcription factor 1 in the differential diagnosis of primary pulmonary carcinoma: evaluation of 1674 cases by tissue microarray. Arch Pathol Lab Med 2012;136:163–71. 10.5858/arpa.2011-0320-OA [DOI] [PubMed] [Google Scholar]
- 18.Moldvay J, Jackel M, Bogos K et al. The role of TTF-1 in differentiating primary and metastatic lung adenocarcinomas. Pathol Oncol Res 2004;10:85–8. 10.1007/BF02893461 [DOI] [PubMed] [Google Scholar]