Abstract
We study the Medicare Part D prescription drug insurance program as a bellwether for designs of private, non-mandatory health insurance markets, focusing on the ability of consumers to evaluate and optimize their choices of plans. Our analysis of administrative data on medical claims in Medicare Part D suggests that fewer than 25 percent of individuals enroll in plans that are ex ante as good as the least cost plan specified by the Plan Finder tool made available to seniors by the Medicare administration, and that consumers on average have expected excess spending of about $300 per year, or about 15 percent of expected total out-of-pocket cost for drugs and Part D insurance. These numbers are hard to reconcile with decision costs alone; it appears that unless a sizeable fraction of consumers place large values on plan features other than cost, they are not optimizing effectively.
Keywords: Medicare, prescription drugs, health insurance demand
1. Introduction
Medicare Part D gives the Medicare-eligible population of seniors access to a subsidized market for non-mandatory standardized prescription drug coverage through contracts sponsored by private insurance firms and approved by the Centers for Medicare and Medicaid Services (CMS). This market, which began operation in 2006, is representative of a trend toward “consumer-directed healthcare” that relies on consumer behavior and competition among insurers and accountable care organizations to attain satisfactory allocation of health care resources with limited government regulation. It is one model for more comprehensive reform of health care insurance (see Newhouse, 2004; Bach and McClellan, 2005; Buntin et al., 2006; Goodman, 2006; and the references therein). Overall, Medicare Part D is considered a success story: Despite a rocky start, enrollment rates are high1, consumers have a broad choice of insurers, and premiums are lower than anticipated by policymakers and insurers (Heiss, McFadden, and Winter, 2006, 2007; Goldman and Joyce, 2008; Duggan, Healy, and Scott-Morton, 2008).2
Despite these successes, making optimal, or even just reasonable, decisions in the Part D market is difficult for seniors. Individuals who are not on employer retirement plans or low-income subsidies have to decide whether to enroll in Part D, and if they do enroll must choose among more than 30 competing stand-alone Prescription Drug Plans (PDP) in most Medicare regions, or alternately Medicare Advantage (MA) plans that bundle a prescription drug benefit with health maintenance coverage. In evaluating plans, individuals face uncertainty with respect to their future health status and drug needs, and rather complicated benefit schedules and formularies, including a coverage gap in most PDP plans and other peculiar institutional features of the Part D program described later in some detail. To navigate these decisions successfully, consumers need to know their current health conditions and drug use, and understand the risk that they will have different drug needs in the future; they need to process this information together with plan features to form realistic expectations regarding drug-related expenditures under alternative plans; and they need to adopt a decision-making protocol that accounts for expected costs and risk, and maximizes their preferences. Failure to choose a good plan could result from violation of any of these elements.
Poor plan choices by seniors can obviously reduce individual welfare, and offset the potential gain from having menus of alternatives. Further, failure of consumers to consistently “vote with their feet” and reject inferior plans blunts the market pressure on insurers to operate efficiently.3 Part D is an informative experiment on how consumers behave in real-world decision situations with a complex, ambiguous structure and high stakes, and may yield predictions for how they will handle plan choices in the new general health insurance exchanges that will implement the Patient Protection and Affordable Care Act of 2010.
The quality of plan choice was considered a major issue from the start of the Part D program (see, e.g., Neuman and Cubanski, 2009). How seniors decide whether to enroll in Medicare Part D and what plans they select determines the impact of CMS policies regarding the entry and offerings of insurers in the Part D market: regulation of formulary and benefit designs (FBD); information provided by the government to consumers, pharmacists, and providers; and regulation of the content and scope of marketing by insurers. CMS has undertaken an extensive program of outreach to provide relevant information and guidance to consumers. In particular, these efforts include its Plan Finder, an internet tool that gives the available plans, premiums, and out-of-pocket costs for any list of prescription drugs (her “medicine cabinet”) specified by the consumer. This tool can help consumers collect and process relevant information on predicted needs, and if consumers pay attention to Plan Finder’s recommendations, remove an important source of poor plan choices. Other flaws in decision-making, such as inattention and procrastination, may require different interventions, for example auto-enrollment with Plan Finder recommendations as defaults while allowing consumers to opt out or override. We return to these issues in the concluding section.
In this paper, we examine how well consumers did in choosing their Medicare Part D insurance plan in the first few years of the program. We use the 20% samples of Medicare claims records that have recently become available for scientific research to construct working samples of Medicare enrollees in stand-alone Part D plans for the years 2006, 2007, and 2008. The size of the dataset – our yearly analysis samples comprise about 1.2 million observations – allows for a great level of detail in the construction of drug use and cost variables. For the individuals in these samples, we compute total drug-related costs for their chosen plans and for all the plans that were available to them. We then calculate the ex ante losses from not choosing optimal plans. For this purpose, we develop an expected utility framework that allows for uncertainty about drug needs in the coming year. We also study alternative decision rules and assumptions with respect to expectations formation.
A number of papers have considered the quality of plan choices in Medicare Part D, but mostly with rather specific or small samples, and also with somewhat inconsistent findings. We briefly review three recent studies, and refer the reader to these papers for additional references.4
Abaluck and Gruber (2011) use comprehensive pharmacy data provided by Wolters Kluwer that cover almost one-third of all third-party prescription drug transactions. They match these data with information on the characteristics of all the plans available to the individuals in the dataset. Abaluck and Gruber find that in their plan choice, individuals place more weight on plan premiums than on expected out-of-pocket costs. Also, individuals value plan financial characteristics in excess of their possible impacts on financial expenses or risk, while placing almost no value on variance-reducing aspects of plans.
Ketcham et al. (2012) analyze a large data set from a “single insurer that sells Part D plans (PDPs) and administers PDPs sold by other companies”. The data contain information on individuals’ chosen and available plans, prescription drug use and spending, and other characteristics. Their analysis focuses on the issue of whether the choices of Medicare Part D enrollees improved over the first two years of the Medicare Part D program in terms of reducing overspending, defined as “the consumers annual ex post out-of-pocket (OOP) costs for insurance and prescription drugs above the cost of the cheapest alternative, where the alternatives include other Part D plans as well as having no coverage”. They find large reductions in ex post overspending from 2006 to 2007, which they attribute mostly to plan switching. These findings contrast with those of Kling et al. (2012) who argue that consumers’ choices are subject to substantial “comparison frictions” and arrive at a more pessimistic conclusion about consumers’ ability to choose the best plans.
Zhou and Zhang (2012) show that in 2009, only 5.2 percent of beneficiaries chose the cheapest Medicare Part D plan (given their current drug needs). They use a 5 percent sample of Medicare Part D claims together with publically available formulary data. Since their approach and data are generally similar to ours but they arrive at slightly different findings, we return to that paper when we present our results in section 4.3.
The remainder of this paper is structured as follows. In Section 2, we describe the data and the approach taken for simulating the relevant attributes of alternative plans available to each consumer. We use Medicare administrative data on drug spending to characterize Part D enrollment decisions in Section 3. In Section 4, we present an analytical framework for analyzing ex ante and ex post optimization failures, along with the results. Section 5 concludes.
2. Data
In this section, we first describe in Section 2.1 the construction of our working samples. Section 2.2 then describes how we simulate the relevant costs consumers face under alternative plans; this includes constructing the plan formularies (which are not publically available in the years of our analysis) from the universe of claims records. We check the validity of these simulations in section 2.3. The use of claims data, the rather complex structure of Medicare Part D, and the lack of publically available formulary information for Part D plans, made these constructions demanding. We document our procedures in some detail; additional information can be found in the appendices.
2.1 Data sources and definition of working samples
This study is based upon the Medicare claims records of a 20 percent representative sample of the approximately 45 million people enrolled in Medicare in the years 2006, 2007, and 2008. Each person enrolled in Medicare has a Health Insurance Claim (HIC) code, which they retain for life (tracked through a change in the HIC code of their primary beneficiary when necessary). The sample, obtained from CMS under a special data use agreement, selects all enrollees with HIC codes ending in ‘0’ or ‘5’; this includes 9,086,340 Medicare beneficiaries in 2006; 9,299,848 in 2007; and 9,530,609 in 2008. People enrolled in any year appear longitudinally across all the years in which they are enrolled by virtue of retaining their HIC code with the same terminal digit. The sample grows each year as new people become eligible to enroll (primarily by reaching age 65 or developing a qualifying disability), and shrinks as people become ineligible (primarily through death or recovery from a qualifying disability).
The sample data include Medicare Parts A and B claims records for 2002–2008, and Part D claims records for 2006–2008, censored to the left for persons who initially enroll in Medicare before 2002, and censored to the right for persons whose enrollment ends after 2008. A major advantage of the claims data is the size and representativeness of the sample; a drawback is that these administrative records contain no clinical data on medical conditions, very limited data on the socioeconomic status of enrollees, and no data on risk preferences, perceptions, or decision-making circumstances that might be used to identify social or behavioral sources of poor plan choices.
To study Part D plan choices of seniors, we form working samples of Part D enrollees who have unrestricted choice among all the plans available in their Medicare region, and have sufficient data on their health, drug use, out-of-pocket costs, and premiums to evaluate the quality of their plan choices. These working samples include 1,139,469 people in 2006, 1,249,301 in 2007, and 1,307,396 in 2008. The working samples screen out (1) persons under age 65, (2) persons who do not qualify for Medicare benefits because they live outside the U.S., (3) persons not enrolled in Part D, (4) enrollees in Medicare Advantage plans, (5) persons who are dual-eligible5 or on low-income subsidies (LIS), (6) persons on retiree plans who do not have unrestricted plan choice, (7) persons who are not enrolled in continuous Part D stand-alone plan coverage throughout the year, (8) persons who are enrolled in employer group waiver (EGWP) plans, and (9) persons who are without prior year claims information (when the analysis requires this information).6
Screens (1) and (2) define our target population; we exclude people under age 65, who are on Medicare due to institutionalization or qualifying disabilities, because their health is quite different from that of the senior population. Screen (3) excludes Part D non-enrollees, and screen (4) excludes Medicare Advantage enrollees, because their claims records to not include comparable data on health conditions and drug use. Screens (5) and (6) exclude people who have restricted choice alternatives and subsidized costs. Enrollees who pass screens (1) through (6) are all enrolled in stand-alone PDP. Screens (7) through (9) exclude people with incomplete information on drug options, cost, or use. Our final working sample records contain Part D enrollment status and (encrypted) plan choice in each of the years 2006, 2007, and 2008, and all prescription drug claims, including medications, benefits paid, total prescription costs, and copayments.
The screens that define our working samples are substantive selections from the overall age 65+ target population. First, they exclude people who (endogenously) choose either no drug coverage or a Medicare Advantage plan that includes drug coverage. Second, they exclude people who have drug coverage through a channel in which they do not see the full premiums, copayments, and alternatives of stand-alone PDP. Third, they exclude people who become newly eligible for Medicare within the year, people who die before the end of an enrollment year, and people who switch into or out of Medicare Advantage coverage voluntarily or as a result of institutionalization. Thus, our working samples under-represent new Medicare enrollees because we have insufficient observed health history to evaluate their choices; this is an issue because initial Plan D choice may have a strong influence on subsequent choices due to inertia and switching costs. Our samples also under-represent terminal enrollees who in the last year of life are likely to incur new health conditions and drug needs. We find that the selected populations from which the working samples are drawn have higher shares of females and whites, and more chronic health conditions, than the overall age 65+ population; this is attributable primarily to the exclusion of people on retiree health plans. Thus, our working samples contain people with relatively well-defined and complete plan choice sets. Conclusions about plan choice behavior from these samples do not necessarily generalize to the overall population of seniors. However, our samples avoid significant self-selection and coverage issues in populations such as the customers of specific pharmacy chains that have been sources of samples used in earlier research.
Appendix A details the selection process, with Table A1 giving the number of people remaining after each screening step, with their average age, the shares of females and of whites, and the average number of chronic health conditions. Because of the screens we apply, the numbers of beneficiaries at various steps in Table A1 do not match up directly with published statistics. Consider the 20 percent sample in 2007. Of the 9,299,848 beneficiaries on the denominator file, 87 percent are identified as having some form of prescription drug coverage at some point in 2007. This is a little less than the 90 percent coverage rate targeted by CMS and achieved in various enrollment reports; the difference may be under-reporting in the denominator file of some forms of coverage that are counted by CMS in total coverage, or exclusion of some denominator file groups in the reporting of coverage rates. The share of the 9,299,848 beneficiaries with Part D coverage at some point in 2007 is 56.1 percent, and with Part D coverage throughout the year is 48.7 percent. These shares exclude individuals with creditable or retiree coverage who are not enrolled in Part D, and individuals who move between stand-alone Part D and other plans (e.g., Medicare Advantage), or who become newly eligible during the year. Table A2 gives a breakdown by age of the patterns of part and full-year Medicare enrollment, and indicates the selection resulting from screening new enrollees and terminal enrollees from our working samples.
Table A1.
Definition and properties of working samples
| Number | % Total | Age | % Female | % White | # chronic conditions | |
|---|---|---|---|---|---|---|
| 2006 | ||||||
| Total | 9,086,340 | 100 | 70.7 | 55.7% | 83.6% | |
| U.S. residents aged 65+ | 7,120,960 | 78 | 75.3 | 57.7% | 85.7% | |
| Enrolled in Part D | 3,764,474 | 41 | 75.6 | 63.2% | 82.3% | |
| Enrolled in standalone Part D Plan (PDP) | 2,639,297 | 29 | 75.8 | 65.3% | 82.4% | 1.68 |
| Non dual-eligible, Non-LIS | 1,466,200 | 16 | 75.0 | 62.5% | 94.2% | 2.68 |
| Non-employer group waiver plan | 1,332,955 | 15 | 75.0 | 63.1% | 94.3% | 2.90 |
| Continuously enrolled | 1,139,469 | 13 | 75.2 | 63.8% | 94.7% | 3.18 |
| Prior year data available | NA | NA | NA | NA | NA | NA |
| 2007 | ||||||
| Total | 9,299,848 | 100 | 70.6 | 55.5% | 83.4% | |
| U.S. residents aged 65+ | 7,235,063 | 78 | 75.3 | 57.5% | 85.5% | |
| Enrolled in Part D | 4,003,149 | 43 | 75.6 | 62.6% | 82.5% | |
| Enrolled in standalone Part D Plan (PDP) | 2,712,376 | 29 | 75.9 | 64.7% | 82.6% | 2.14 |
| Non dual-eligible, Non-LIS | 1,535,704 | 17 | 75.0 | 62.1% | 93.8% | 3.35 |
| Non-employer group waiver plan | 1,418,070 | 15 | 75.0 | 62.6% | 94.1% | 3.57 |
| Continuously enrolled | 1,249,301 | 13 | 75.2 | 63.4% | 94.6% | 3.83 |
| Prior year data available | 1,147,353 | 12 | 75.4 | 64.0% | 94.8% | 3.93 |
| 2008 | ||||||
| Total | 9,530,609 | 100 | 70.5 | 55.3% | 83.2% | |
| U.S. residents aged 65+ | 7,403,722 | 78 | 75.2 | 57.3% | 85.4% | |
| Enrolled in Part D | 4,215,955 | 44 | 75.5 | 62.0% | 82.7% | |
| Enrolled in standalone Part D Plan (PDP) | 2,749,743 | 29 | 75.8 | 64.2% | 82.8% | 2.46 |
| Non dual-eligible, Non-LIS | 1,589,870 | 17 | 75.0 | 61.5% | 93.8% | 3.48 |
| Non-employer group waiver plan | 1,450,372 | 15 | 75.0 | 62.1% | 94.2% | 3.74 |
| Continuously enrolled | 1,307,396 | 14 | 75.2 | 63.0% | 94.6% | 4.02 |
| Prior year data available | 1,230,347 | 13 | 75.4 | 63.5% | 94.7% | 4.10 |
Table A2.
Medicare enrollment by age in 2007
| Age January 2007 | stayers | entrants | leavers (death) | Leavers (other) | entrants and leavers (other) | entrants and leavers (death) |
|---|---|---|---|---|---|---|
| Start: | January | After January | January | January | After January | After January |
| Stop: | December | December | Before December | Before December | Before December | Before December |
| 64 | 98,214 | 393,088 | 2,704 | 24 | 71 | 1,435 |
| 65 | 444,480 | 4,720 | 5,934 | 88 | 17 | 9 |
| 66 | 421,186 | 2,016 | 6,198 | 79 | 9 | 6 |
| 67 | 402,074 | 1,243 | 6,193 | 70 | 11 | 7 |
| 68 | 395,671 | 972 | 6,753 | 77 | 5 | 3 |
| 69 | 374,095 | 831 | 6,985 | 66 | 5 | 3 |
| 70 | 355,531 | 602 | 7,258 | 58 | 7 | 6 |
| 71 | 345,095 | 535 | 7,784 | 77 | 9 | 2 |
| 72 | 330,193 | 435 | 8,298 | 86 | 6 | 2 |
| 73 | 306,904 | 355 | 8,434 | 73 | 5 | 1 |
| 74 | 306,732 | 357 | 9,154 | 80 | 5 | 4 |
| 75 | 295,700 | 324 | 9,600 | 86 | 2 | 4 |
| 76 | 293,876 | 250 | 10,578 | 102 | 8 | 2 |
| 77 | 273,060 | 221 | 10,660 | 76 | 7 | 5 |
| 78 | 265,997 | 211 | 11,437 | 82 | 2 | 2 |
| 79 | 256,164 | 146 | 12,335 | 87 | 0 | 4 |
| 80 | 237,797 | 125 | 12,633 | 91 | 4 | 2 |
| 81 | 223,355 | 124 | 13,321 | 70 | 0 | 2 |
| 82 | 210,338 | 99 | 13,925 | 73 | 1 | 4 |
| 83 | 189,343 | 81 | 13,754 | 77 | 0 | 2 |
| 84 | 171,297 | 56 | 13,977 | 75 | 2 | 2 |
| 85 | 157,259 | 65 | 14,348 | 63 | 0 | 2 |
| 86 | 134,761 | 41 | 13,946 | 57 | 0 | 2 |
| 87 | 108,484 | 39 | 12,474 | 45 | 1 | 0 |
| 88 | 95,107 | 23 | 12,449 | 36 | 0 | 1 |
| 89 | 77,746 | 23 | 11,273 | 25 | 1 | 0 |
| 90+ | 295,594 | 63 | 60,510 | 41 | 0 | 3 |
Source: Tabulations from 20% sample of Medicare enrollees
For people in the 20 percent sample facing a plan choice in an end-of-year enrollment period, we use indicators of health conditions from the Chronic Condition Warehouse classification and coding provided by CMS, described in Appendix A. Table A3 lists these health conditions, and their prevalence in the working samples; these are basis for the counts of health conditions that appear in Table A1.7
Table A3.
Health conditions and working sample prevalence
| Chronic condition | Reference Time Period (# years) | Number/Type of Claims to Qualify | Prevalence | ||
|---|---|---|---|---|---|
| 2006 | 2007 | 2008 | |||
| Acute Myocardial Infarction | 1 | 1 inpatient claim | 2.0% | 2.8% | 3.1% |
| Alzheimer’s Disease | 3 | 1 inpatient or HOP/carrier claim | 2.4% | 3.5% | 3.6% |
| Alzheimers Disease, Senile Dementia, Related Disorders | 3 | 1 inpatient or HOP/carrier claim | 5.5% | 7.8% | 8.1% |
| Atrial Fibrillation | 1 | 1 inpatient or 2 HOP/Carrier claims | 9.7% | 12.5% | 12.9% |
| Breast Cancer | 1 | 1 inpatient or 2 HOP/carrier claims | 3.5% | 4.3% | 4.5% |
| Cataract | 1 | 1 HOP/carrier claim | 49.4% | 59.0% | 61.2% |
| Chronic Kidney Disease | 2 | 1 inpatient or 2 HOP/carrier claims | 7.0% | 10.5% | 12.2% |
| Chronic Obstructive Pulmonary Disease | 1 | 1 inpatient or 2 HOP/carrier claims | 12.5% | 16.3% | 17.1% |
| Colorectal Cancer | 1 | 1 inpatient or 2 HOP/carrier claims | 1.5% | 2.0% | 2.1% |
| Depression | 1 | 1 inpatient or HOP/carrier claim | 12.7% | 16.9% | 18.2% |
| Diabetes | 2 | 1 inpatient or 2 HOP/carrier claims | 20.6% | 26.1% | 27.5% |
| Endometrial Cancer | 1 | 1 inpatient or 2 HOP/carrier claims | 0.3% | 0.4% | 0.5% |
| Glaucoma | 1 | 1 carrier claim | 14.8% | 18.0% | 18.9% |
| Heart Failure | 2 | 1 inpatient or HOP/carrier claim | 14.3% | 19.1% | 19.7% |
| Hip/Pelvic Fracture | 1 | 1 impatient claim | 1.5% | 2.2% | 2.4% |
| HIV/AIDS | 1 | 1 inpatient or HOP/carrier claim | 0.0% | 0.0% | 0.0% |
| Hypertension | 1 | 1 inpatient or HOP/carrier claim | 64.6% | 76.6% | 77.6% |
| Ischemic Heart Disease | 2 | 1 inpatient or HOP/carrier claim | 32.6% | 40.4% | 41.7% |
| Lung Cancer | 1 | 1 inpatient or 2 HOP/carrier claims | 0.6% | 0.9% | 1.0% |
| Osteoperosis | 1 | 1 inpatient or HOP/carrier claim | 23.8% | 29.3% | 30.9% |
| Prostate Cancer | 1 | 1 inpatient or 2 HOP/carrier claims | 3.7% | 4.5% | 4.8% |
| Rheumatoid Arthritis/Osteoarthritis | 2 | 2 inpatient or HOP/carrier claims | 21.9% | 28.0% | 30.0% |
| Stroke | 1 | 1 inpatient or 2 HOP/carrier claims | 8.0% | 10.7% | 11.3% |
Source: Chronic Condition Warehouse, http://www.ccwdata.org/chronic-conditions/index.htm for the diagnostic codes assigned to each health condition. For HIV/AIDS which is not coded by CCW, we use ICD-9 DX codes 042XX 043XX 044XX 07953 V08XX; these are taken from CMS’s HCC risk model.
A key variable in our analysis is an individual’s total drug bill (TDB) in her chosen stand-alone Part D plan, defined as the annual sum of pharmacy prices of her prescriptions (her medicine cabinet) reported in her Part D claims files.8 Figure 1 gives the cumulative distribution functions of 2006, 2007, and 2008 total drug bills in our final working samples. These distributions are virtually identical, even though the bills are in nominal dollars, indicating that trends in pharmacy benefit management, changes in drug prices and the patent status of some major branded drugs, and changes in the drug needs of the population in stand-alone PDP coverage have effectively cancelled each other out. Also shown in Figure 1 is CMS’s forecast in 2002 of the distribution of 2006 drug bills, based on the 1998 Medicare Current Beneficiary Survey, which was used by government agencies and some insurers to predict the costs of providing Part D coverage. In retrospect, this forecast substantially over-estimated the mean drug bill and the dispersion of drug bills. The 2002 forecast had a mean of $2732 (s.d. $3395) compared with a mean of $2050 (s.d. $2146) in the 2006 realization. The descriptive statistics for the realized total drug bills in 2006, 2007, and 2008 are reported in Table 1. The table also shows the correlations of drug bills across years, which are fairly high (above to 75% for adjacent years). We return to these numbers when we describe enrollment choices in section 3 below.
Figure 1.
Cumulative distribution functions of annual total drug bills
Table 1.
Descriptive statistics for observed total drug bills
| 2006 | 2007 | 2008 | |
|---|---|---|---|
| Mean | $2050 | $2258 | $2299 |
| Median | $1679 | $1831 | $1811 |
| Percent Zero | 6.1% | 3.7% | 3.5% |
| s.d. | $2146 | $2551 | $2906 |
|
| |||
| Correlations | |||
| TDB 2006 | 0.768 | 0.631 | |
| TDB 2007 | 0.797 | ||
Note: All figures are in current dollars.
2.2 From prescription drug claims data to simulated plan choices
For each beneficiary in our working samples in each of the years 2006, 2007, and 2008, we observe a complete record of each prescription filled and submitted to Medicare for reimbursement. Each claim includes detailed information on the pharmacy price9 and copayment for the particular drug and quantity dispensed, days supplied, on which tier the insurance plan classifies the drug, the benefit phase associated with each claim, the National Drug Classification (NDC) code of the drug, and the prescription’s date. We also observe for each beneficiary the critical determinant of benefit phase in Part D plans, the cumulative true out-of-pocket (TrOOP) cost in a benefit year, defined by CMS as enrollee out-of-pocket payments for drugs contained in a plan’s formulary (including drugs covered on appeal).
We assume that consumers care about annual out-of-pocket (AOOP) costs, which we define as annual TrOOP costs plus over-the-counter and off-formulary prescription drug purchases that do not generate claims, and about annual total drug-related expenditures, the sum of annual plan premiums and AOOP costs, which we term consumer inclusive cost (CIC).10 However, we have no data on drug purchases that do not generate claims. Therefore, for chosen plans we approximate AOOP costs by annual TrOOP costs and CIC (the sum of premium and AOOP) by the sum of premium and TrOOP costs. For people who purchase drugs that do not generate claims, these approximations will understate total drug-related expenditures and miss some medications.
Our study of choice behavior starts from a simulation of each beneficiary’s annual TrOOP and AOOP costs, and CIC, for each available stand-alone Part D plan in his region. There are two main parts to this simulation: (1) determining the formulary and benefit design (FBD) for each plan, and (2) running each beneficiary through these FBDs to estimate annual TrOOP and AOOP costs, and CIC, based on each plan’s rules and copayment provisions.
2.2.1 Construction of empirical FBDs
There are five types of Part D stand-alone PDPs approved by CMS: Standard, Actuarially Equivalent, Basic Alternative, Generic Extended, and Full Extended. A Standard plan has an administratively specified benefit schedule with four phases determined by an annual deductible, an initial coverage phase with a 25 percent copayment, a gap or doughnut hole with no coverage when TrOOP is between the level determined by an initial coverage limit (ICL) and a catastrophic coverage threshold (CCT), and a catastrophic phase above the CCT with a 5 percent (or equivalent) copayment. Table 2 gives the Standard plan benefit parameters for each year in our data, and the drug price index used by CMS to adjust these parameters each year. Actuarially Equivalent plans differ from the Standard plan only by substituting copayment tiers for copayment percentages, keeping benefit generosity the same on average. Basic Alternative plans are like Actuarially Equivalent plans, but eliminate the deductible phase, and are required to be at least as generous as the Standard plan. Enhanced plans resemble Basic Alternative plans, but add gap coverage at the equivalent of a 25 percent copayment rate, for generic drugs only in Generic Enhanced plans, and for all formulary drugs in Full Enhanced plans. Because Enhanced plans reduce OOP costs through the gap phase, higher drug bills are required to reach the CCT.11
Table 2.
Characteristics of Medicare Part D plans
Characteristics fixed by plan
|
Characteristics specific to drugs within a plan
|
| Characteristics established by Medicare standard plan parameters | |||
|---|---|---|---|
| 2006 | 2007 | 2008 | |
| Deductible | $250 | $265 | $275 |
| TrOOP at Initial Coverage Limit (ICL) | $1,750 | $1,866 | $1,951 |
| Initial Coverage Limit | $2,250 | $2,400 | $2,510 |
| TrOOP limit for catastrophic coverage | $3,600 | $3,850 | $4050 |
| Drug bill for catastrophic coverage | $5,100 | $5451.25 | $5,726.75 |
| Copayment, Deductible < Bill < ICL | 25% | 25% | 25% |
| Copayment, catastrophic coverage: | |||
| Generic or Preferred Multi-Source | $2.00 | $2.15 | $2.25 |
| Other Drugs | $5.00 | $5.35 | $5.60 |
| Drug Cost Index | 1.000 | 1.067 | 1.116 |
The Part D data identify plan types, but CMS confidentiality rules encrypt the identities of individual plans. Consequently, we cannot assign published plan formularies from public CMS records to these encrypted identifiers, and thus are unable to calculate from actual formularies the benefits and out-of-pocket costs for plans available but not chosen.12 As a substitute, we construct an empirical formulary for each insurance plan that is the union of all the NDC codes of claims of enrollees in our Part D working sample who are in plans with the same formulary identifier in a specified year. Appendix B details this construction, and its limitations.
Our data give, for each drug appearing in the empirical formulary of a plan, its branded/generic/preferred status classification, tier classification (with processing of off-tier claims detailed in Appendix B), and pharmacy price (total cost). We estimate a benefit design for each plan based on drug classification, phase-dependent empirical tier copayment rate, days supplied, and the type of pharmacy that fills the prescription. At the end of this construction, we have an empirical FBD for each plan and year that we use to estimate the annual TrOOP cost of any specified list of prescriptions supplied under that plan.
2.2.2 Simulation of TrOOP and AOOP costs, and CIC, for alternative plans
Each person in our working sample is characterized by an observed medicine cabinet (MC) – the list of prescriptions a person has filled over a year – which we obtain from her claims records. As noted earlier, observed medicine cabinets exclude over-the-counter and off-formulary prescription drugs that do not generate claims. In making plan choices, a person may consider their medicine cabinet in the previous year and other “what if” medicine cabinets that they might require contingent on future health conditions. To study the quality of plan choices, we impute for various medicine cabinets “what if” AOOP costs in the available plans, both chosen and alternative. To do this, we plug each medicine cabinet into the empirical FBD for each available plan to estimate TrOOP costs, add in the pharmacy price of drugs in the medicine cabinet that are not in the plan’s formulary to estimate AOOP costs, and finally add in annual premiums to estimate CIC.
Several important behavioral assumptions enter this estimation, and affect the interpretation of choice quality. First, we assume that over-the-counter and off-formulary drug purchases in a chosen plan continue to be made under any alternative plan, so that in plan comparisons these purchases wash out. However, it is possible that some off-formulary drug purchases under a person’s chosen plan will be covered at lower net out-of-pocket cost in an alternative plan, in which case we overstate the net AOOP difference between the alternative and chosen plan. Second, we make the no-substitution assumption that the medicine cabinet will not be modified under alternative plans, so that exactly the same drugs and dosages are used. If instead the person adjusts their medicine cabinet to the alternative plan, substituting therapeutically equivalent drugs and/or adjusting dosages to obtain lower copayments, then our estimation will again overstate the net AOOP difference between the alternative and chosen plans. Then, under these behavioral assumptions, our analysis may conclude incorrectly that a chosen plan is superior to an alternative plan, but should never conclude incorrectly that a chosen plan is inferior to an alternative plan.
To test the no substitution assumption, we also consider a therapeutic substitution assumption. Under that alternative, if a medicine cabinet contains a drug that is not in the formulary of one of the available alternative plans, this drug is replaced in all plans, including the chosen plan, by a therapeutic equivalent with the lowest copayment. In general, this may understate actual AOOP in the chosen plan, but the estimation allows an “apples-to-apples” comparison of plan costs. However, it is possible that if a person prefers a specific drug to a lower cost therapeutic equivalent in their chosen plan, and another plan does not have this specific drug in their formulary but has a lower cost therapeutic equivalent, our analysis could incorrectly conclude that the chosen plan is inferior to this alternative plan. If medicine cabinets are predetermined by patient and provider preferences, then the no substitution assumption is the most appropriate. However, if medicine cabinets are endogenously determined by patients and providers interacting with pharmacy benefit managers and insurers, then the therapeutic substitution assumption is more appropriate. Details of the estimation of TrOOP, AOOP, and CIC for alternative plans and medicine cabinets are given in Appendix C.
2.2.3 Validity of the simulation
The simulation’s internal validity is tested by examining simulated minus actual AOOP spending, with no substitution in the simulation, for each beneficiary in their chosen plan. Actual AOOP is defined here as the sum of patient payments not reimbursed by a third party, all qualified third party payments, and patient liability reductions due to coordination of benefits from other payers. The median difference in simulated minus actual AOOP is $0, the mean difference is −$13.62, and the correlation coefficient exceeds 0.99. This compares favorably to a similar simulation check presented in Ketcham et al. (2012). Figure 2 displays the distribution of this difference; for more than 99.6 percent of the beneficiaries, the difference is less than $500 in absolute value. The small size of these differences for most beneficiaries suggests that the simulation performs reasonably well and is likely accurate for predicting AOOP spending in the plans not chosen.
Figure 2.
Distribution of differences between actual and simulated OOP costs
However, there are still some outliers for whom the difference between simulated and actual AOOP is large. Figure 3 plots the empirical mean of simulated AOOP cost in 2007 against overall drug bill for consumers on various plans, and compares this with the designed standard plan benefit schedule. The figure indicates that the simulated benefits for Standard and Actuarially Equivalent plans conform well to the designed schedule over the phases where benefits are paid, but give somewhat higher AOOP costs when drug bills are in the gap. This may be a statistical artifact, or may be evidence that consumers are being surcharged on drugs purchased in the gap where there is no coverage. The AOOP costs for plans with brand and generic gap coverage show the expected reduction of AOOP costs in the gap phase. The relatively higher AOOP costs for plans covering only generics in the gap phase may suggest that branded drugs are the primary cost driver at that level of spending. For comparability of chosen and non-chosen plans, we use hereafter annual imputed adjusted AOOP costs of a medicine cabinet, with or without therapeutic substitution, rather than a mix of observed AOOP costs for the chosen plan and imputed AOOP costs for alternative plans.
Figure 3.
Empirical means of 2007 simulated out-of-pocket (OOP) costs for alternative plan types and the Part D standard plan designed benefit schedule, conditioned on annual total drug bill
3. Enrollment choices
We begin our analysis of the administrative data on Medicare Part D by looking at the enrollment decision; this complements earlier research that used survey data such as Winter et al. (2006) and Heiss et al. (2006, 2009).
The Part D program is heavily subsidized, with insurers reimbursed from government general revenues for about 75 percent of overhead and benefits paid out, with fairly tightly regulated formulary and benefit design and competitively determined premiums. As a result, the program is first-year actuarially favorable for most eligible people, even before considering the value of reducing risk and the option value of avoiding delayed enrollment penalties if a Part D plan becomes attractive in the future (Winter et al., 2006).
The marketing and information provided on Part D policies by insurers and by CMS focus on the expected benefits rather than on risk reduction. In particular, the CMS Plan Finder invites users to list current drugs, and then provides a list of available plans ranked by out-of-pocket cost if the current drug use continues through the coming year. While consumers could in principle use Plan Finder on a “what if” basis by introducing counterfactual drugs and dosages, it would be cumbersome to do this and combine the results into an analysis of expected plan benefits and costs. No direct information is provided on the likelihood that the person will have different drug needs, the ability of plans to meet these needs, and the reduction in risk offered by plans with more generous coverage. As a result, consumers are nudged toward lowest cost plans under the static forecast that current drug use will continue without change, rather than being nudged toward overall risk management.
This said, the set of current drugs taken and the total drug bills (TDB) are good predictors of one-year-ahead drug needs. Table 1 above shows that the pairwise correlations of TDB between adjacent years over the period 2006, 2007, and 2008 are all above 0.75; the table also provides descriptive statistics for each year. These statistics suggest that persons with even modest drug bills can expect to be ahead of the game by enrolling, even if they are not risk-adverse or concerned about the late enrollment penalty and future options. As noted above, the cumulative distribution functions of 2006, 2007, and 2008 total drug bills for the populations that had full-year enrollment in a stand-alone prescription drug plan in all three years, depicted in Figure 1, are virtually identical in 2007 and 2008, and only slightly shifted downward in 2006, even though bills are in nominal rather than real terms. This indicates that adjustments in pharmacy benefit management have essentially offset rising drug prices, changing patent status of some major branded drugs, and changing risk characteristics of the population that selects stand-alone PDP coverage. Also plotted in Figure 1 is the 2008 density, scaled, which shows a piling up of people at the beginning of the gap where increased copayments reduce incremental demand for drugs.13
Figure 4 gives the cumulative distribution functions for 2008 total drug bills, conditioned on the 2007 drug bill percentiles. As the relatively high inter-year correlation implies, these CDF are relatively tightly distributed around the 2007 levels, but with some regression to the mean. However, they have relatively thick right tails. Figure 5 plots the conditional mean of the 2008 total drug bill, and the contour giving the approximate 95th percentile, against the 2007 total drug bill. The scales are logarithmic, so that this graph shows substantial risk of large increases in drug bills over the previous year. Below a 2007 drug bill of $2317 (the 71st percentile), the 2008 conditional mean exceeds the 2007 drug bill, and above this level, the reverse is true, reflecting regression to the mean.
Figure 4.
Cumulative distribution functions of 2008 total drug bill conditioned on 2007 total drug bill percentile
Figure 5.
Mean and 95th percentile of 2008 total drug bill, conditioned on 2007 total drug bill
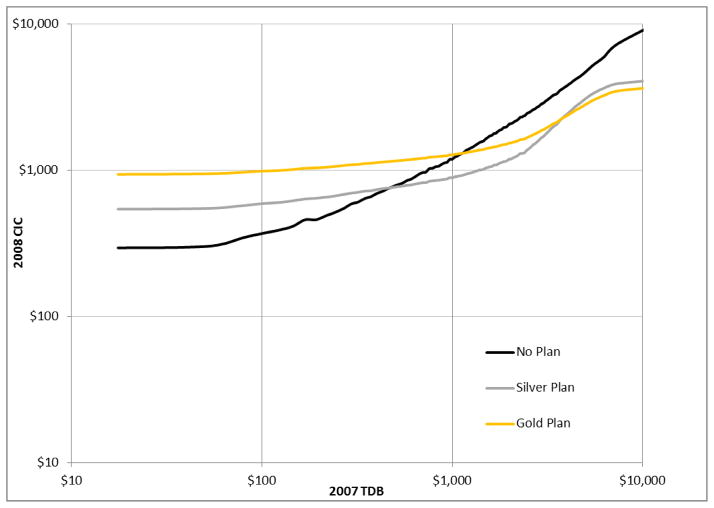
A myopic consumer who considers only first-year benefits from a Part D plan and is risk neutral should enroll if expected benefits received exceed the premium. Consider a Part D standard or equivalent plan in 2008, which had a typical premium of $30 per month. Figure 6 gives the probability, conditioned on 2007 total drug bill, that an enrollee in the standard plan will be ahead of the game in the first year, with benefits exceeding the annual premium. For 2007 drug bills above $690, this probability exceeds 50 percent. The probability peaks at a 2007 drug bill of about $5000, and thereafter declines slightly, apparently due to elevated mortality risk for people with very high drug bills. Figure 7 gives the expected cost conditioned on the 2007 total drug bill for non-enrollment, enrollment in a Silver (i.e., standard or equivalent) plan, and enrollment in a Gold (i.e., generic drug coverage in the gap) plan with a premium at the national average of $63.34 per month. These curves assume that 50 percent of drug costs in the gap are generic. At a 2007 drug bill of $470, corresponding to a 2008 expected drug bill of $760, enrollment in a Silver plan breaks even with non-enrollment in terms of expected cost. Then, if risk aversion and an option value for avoiding a late enrollment penalty in future years are not considerations, 19.5 percent of the eligible population is best off not enrolling. At a drug bill of $3,744 in 2007, corresponding to an expected drug bill of $3,570 in 2008, the Silver and Gold plans break even in terms of expected cost. Then, the 9.5 percent of the eligible population with the highest 2007 drug bills is best off with a Gold plan. Moreover, if first-year payoff is the only criterion and risk is not a consideration, about 12 percent of those enrolling in Part D plans would choose a Gold plan.
Figure 6.
Probability that standard plan enrollment in 2008 at a $30 per month premium gives lower consumer cost than non-enrollment, conditioned on total drug bill in 2007 (log scale)
Figure 7.
2008 expected consumer inclusive cost (OOP plus premium) of “No Plan”, “Silver” (Standard) plan, and “Gold” (gap generic coverage) plan choices, given 2007 total drug bill
In an earlier analysis of Part D enrollment choices using a national sample of about 2,500 eligible people, Heiss, McFadden, and Winter (2009) found that 23.8 percent of those not automatically enrolled in Part D through retiree plans or Medicaid chose to not enroll. CMS tabulations from the denominator file show that 15.1 percent of the eligible population was without drug insurance of some creditable14 form in 2008 – this will translate into a higher percentage of those who are active deciders with a personal choice of whether to enroll and if so what plan to choose (see Table 3).15 While these rates bracket the 19.5 percent that the simple analysis above would suggest if consumers are myopic and risk-neutral, the actual pattern of non-enrollment was much more random, including many who left money on the table in the first year as the result of their enrollment choice. The earlier finding was that non-enrollment was concentrated among those with low but above-poverty incomes, low education, and relatively low prior drug use. The earlier analysis also found that when the option value of Part D insurance without a late enrollment penalty is taken into account, only a few percent of very old people with little drug use should rationally choose to not insure. Then, the observed rates of non-enrollment indicate that myopia and inattention are significant, and are reducing prescription drug insurance participation rates below levels that are optimal for individuals.
Table 3.
Medicare Part D enrollment in December, 2006–2008 (Denominator File)
| 2006 | 2007 | 2008 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | 65+ | Pct | Total | 65+ | Pct | Total | 65+ | Pct | |
| Total | 43,338,571 | 36,316,594 | 44,263,111 | 36,965,846 | 45,411,883 | 37,896,079 | |||
| No Part D, Retiree Drug Subsidy, or Creditable Coverage | 7,801,239 | 6,516,882 | 17.9% | 7,053,805 | 5,799,802 | 15.7% | 6,980,480 | 5,726,326 | 15.1% |
| Part D Enrolled | |||||||||
| Total | 22,854,973 | 18,368,305 | 50.6% | 24,477,276 | 19676031 | 53.2% | 25844675 | 20,790,368 | 54.9% |
| With Creditable Coverage | 2,663,377 | 2,172,276 | 6.0% | 2,922,694 | 2,406,994 | 6.5% | 2,174,420 | 1,831,963 | 4.8% |
| Without Creditable Coverage | 20,191,596 | 16,196,029 | 44.6% | 21,554,582 | 17,269,037 | 46.7% | 23,670,255 | 18,958,405 | 50.0% |
| Retiree Drug Subsidy | |||||||||
| Total | 6,838,613 | 6,552,456 | 18.0% | 7,009,702 | 6,715,950 | 18.2% | 6,655,834 | 6,380,143 | 16.8% |
| With Creditable Coverage | 676,496 | 637,207 | 1.8% | 703,149 | 659,076 | 1.8% | 624,879 | 584,305 | 1.5% |
| Without Creditable Coverage | 6,162,117 | 5,915,249 | 16.3% | 6,306,553 | 6,056,874 | 16.4% | 6,030,955 | 5,795,838 | 15.3% |
| Creditable Coverage (No Part D or Retiree Drug Subsidy) | 5,843,746 | 4,878,951 | 13.4% | 5,722,328 | 4,774,063 | 12.9% | 5,930,894 | 4,999,242 | 13.2% |
| Creditable Coverage Without Regard to Part D or Retiree Drug Subsidy | 9,183,619 | 7688434 | 21.2% | 9348171 | 7840133 | 21.2% | 8730193 | 7415510 | 19.6% |
Source: Centers for Medicare and Medicaid 2007–2009 Statistical Supplements, Table 14.4
We summarize Part D plan choice in our 20 percent sample of all people eligible for Medicare Part D in Table 4. Gold plans (combined with the relatively unimportant Platinum plans with full gap coverage) have a 8.1 percent share of those enrolled in Part D plans in 2007.16 Thus, Gold plans appear to be undersubscribed relative to their first-year actuarial value. The complexity of the valuation of Silver and Gold plans, the availability of plans at national average premiums, consumer errors in assessing the actuarial value of gap coverage and focus on premium costs over potential benefits, and our assumption on the share of generics in gap purchases are factors that may contribute to this difference in predicted and actual market share. What the numbers indicate is that risk aversion is apparently not strong enough to offset the (perceived) disadvantageous loading of extended benefits.
Table 4.
Counts by Part D enrollment status of 20% sample and working sample
| 2006 | % | 2007 | % | 2008 | % | |
|---|---|---|---|---|---|---|
| Panel A. 20% Sample: Part D Enrollment Status in December | ||||||
| Not enrolled in Part D with creditable coverage | 1,295,192 | 14.3 | 1,292,191 | 13.9 | 1,312,470 | 13.8 |
| Not enrolled in Part D without creditable coverage | 2,769,583 | 30.5 | 2,685,661 | 28.9 | 2,630,485 | 27.6 |
| Enrolled in Medicare Advantage plan | 1,311,554 | 14.4 | 1,507,154 | 16.2 | 1,724,249 | 18.1 |
| Enrolled in employer-provided retiree plan (starting Jan 2007) | NA | NA | 25,024 | 0.3 | 25,349 | 0.3 |
| Enrolled in Part D stand-alone basic (silver) plan | 3,121,961 | 34.4 | 3,136,343 | 33.7 | 3,223,848 | 33.8 |
| Enrolled in Part D stand-alone enhanced (gold) plan | 93,125 | 1.0 | 253,080 | 2.7 | 236,295 | 2.5 |
| Enrolled in Part D stand-alone full gap coverage (platinum) | 95,856 | 1.1 | 23,468 | 0.3 | 75 | 0.0 |
| Enrolled in Part D stand-alone plan for which coverage could not be determined | 24,410 | 0.3 | 1 | 0.0 | 1 | 0.0 |
| NEC | 374,659 | 4.1 | 376,926 | 4.1 | 377,837 | 4.0 |
|
| ||||||
| Total, 20% Sample | 9,086,340 | 100 | 9,299,848 | 100 | 9,530,609 | 100 |
|
| ||||||
| Panel B. Working Sample Enrollment throughout year | ||||||
| Enrolled in Part D stand-alone basic (silver) plan | 1,106,503 | 89.8 | 1,062,131 | 84.1 | 1,113,201 | 86.7 |
| Enrolled in Part D stand-alone enhanced (gold) plan | 81,748 | 6.6 | 182,897 | 14.5 | 171,110 | 13.3 |
| Enrolled in Part D stand-alone full gap coverage (platinum) | 44,380 | 3.6 | 18,280 | 1.4 | 45 | 0.0 |
|
| ||||||
| Total, Working Sample | 1,232,631 | 100 | 1,263,308 | 100 | 1,138,105 | 100 |
Note: The top panel displays the enrollment status in December of the particular year, providing a snapshot for all beneficiaries on the denominator file. The bottom panel tabulates the type of gap coverage for our working sample, which is constructed as shown in Table 3. Since our sample is restricted to people enrolled in the same PDP plan throughout the year, these coverage counts remain constant throughout the year.
The analysis above did not condition on the demographic variables available in claims data, gender and age. We find that drug use does vary with these variables, but that conditioned on prior drug use, they have little explanatory power. Thus, further conditioning on these demographic variables does not alter our general conclusions on enrollment.
4. Models of plan choice behavior
In this section, we develop an analytic framework for assessing the quality of Part D plan choices. Plan choices are ex ante, made during the open-enrollment period at the end of each year for the plan that will be in place during the coming year, before actual health conditions and drug needs in the coming year are realized. Evaluations of the quality of plan choice should be correspondingly ex ante, based on expected utility in the year ahead given current information, the decision criterion for rational individuals facing uncertain prospects. We apply this framework to evaluate peoples’ plan choices for 2007 made at the end of 2006, and choices for 2008 made at the end of 2007.
In overview, we ask whether people, using the information they arguably had available at the time of their actual plan choice, could have achieved higher expected utility than they did. We make these assessments by comparing the expected utility of actual choices with the expected utility from benchmark decision rules. A leading benchmark, and the focus of much of our empirical analysis, is the recommendation of the CMS Plan Finder, which ranks the cost of plans in the coming year when given a person’s medicine cabinet for the past year. This decision rule is available to any senior who can access the Medicare website, personally or through a helper. Another benchmark we use in order to make comparisons to the earlier literature is the infeasible rule that maximizes utility under perfect foresight on future health conditions and drug needs. In section 4.1, we give the foundation for the expected utility criterion and provide some preliminary evidence on the elements that enter utility. In section 4.2, we discuss notions of ex ante and ex post optimization errors, our framework for ex ante assessment of the quality of plan choice, and the benchmark decision rules we consider. We present our results on the quality of Part D plan choices in Section 4.3.
4.1 Rational expectations and decisions
We describe a model of expected utility of plans in the Medicare Part D market that allows for uncertainty about future drug needs, and alternative protocols for expectation formation. This model cannot be made operational without a number of assumptions, but it nevertheless provides a framework for forward-looking choices that is consistent with the economic standard for rational choice.
We make the reasonable assumption that at the time of their plan choice at the end of a year for the upcoming year, consumers know the drugs they have used over the current year, their health conditions, and their realized drug bill for the year, and they can calculate from public information (e.g., the CMS Plan finder) their projected CIC for each plan alternative they face and each medicine cabinet they may need. Since the open enrollment periods for 2007 and 2008 were mid-November to mid-December in the preceding year, this is a good but not perfect assumption, as end-of-year events that appear in our information measures may not be predictable by the individual, and no public source including Plan Finder makes it easy to carry through a sophisticated forecast of the likelihood and consequences of changes in health and drug needs.
We assume in practice that the information each consumer has in year t-1, denoted Xt-1, includes their age and gender, their medicine cabinet (MCt-1, a high-dimensional vector that describes all the consumer’s prescription claims over the year and includes information on the drug, dosage, and number of days supplied for each prescription), their end-of-year health status (based on the CMS Chronic Condition Warehouse (CCW) inventory of chronic health conditions), their risk score for expected drug costs (based on the Hierarchical Condition Code (HCC) used by CMS for risk adjustment), their total drug bill, their Part D plan (if any), and the annual premium and realized AOOP cost (and hence realized CIC) under this plan. Of course, it is possible that people cannot retrieve all this information, or that they do so with error. It is also possible that people have additional (private) information on their circumstances, in which case decisions based only on the information above are “boundedly” rational.
Each consumer is assumed to have sufficient information, from the CMS Plan Finder or otherwise, to determine the formulary and benefit design mapping for each Plan k available in year t in her region, which we denote AOOP = FBD(MC,k, t), that gives the AOOP for any given medicine cabinet MC.17 She is also assumed to form, using information obtained from peers or otherwise, expectations of future drug use, i.e., a personal conditional density f(MC | k,Xt-1) of year t medicine cabinets given end-of-year t-1 information. From this, we assume that the consumer can deduce the conditional distribution of AOOP cost in period t given covariates Xt-1 and plan k, as well as its conditional cumulant generating function m, mean MAOOP, and variance VAOOP:
For a consumer with rational expectations, the density f will be statistically accurate. Consumers with less than fully rational expectations may have densities f that are not fully accurate because they ignore available conditioning information, or because their beliefs are not realistic.
We assume that the preferences of consumers can be approximated by CARA utility functions U = (1 − exp(−α(w − Premium − AOOP)))/α, where α > 0 is a coefficient of risk aversion that approaches zero in the limiting case of risk neutrality, w is “permanent income” that can vary across individuals due to variations in wealth and in other risky opportunities, Premium is the annual Part D premium, and AOOP is the (uncertain) annual out-of-pocket cost. Expected utility of plan k in period t given the information Xt-1 available at the time of plan choice is then
When α is small, m(α | k,Xt-1)/α ≈ MAOOP(k, Xt-1) + αVAOOP(k,Xt-1)/2 is a certainty-equivalent expected AOOP. Le ECICkt = Premiumkt + MAOOP(k,Xt-1) denote expected CIC. Then expected utility is a decreasing function of certainty-equivalent expected CIC: ECICkt + αVAOOP(k,Xt-1)/2. Plan choice to minimize this sum in k is then a benchmark for rational choice, and certainty-equivalent expected CIC is a metric for losses when choice behavior is not fully rational. Choice will fail to be fully rational for a consumer with small α if (1) Xt-1 does not include all available relevant information, (2) information processing to calculate MAOOP(k,Xt-1) and VAOOP(k,Xt-1) is statistically inaccurate, or (3) plan choice fails to minimize certainty-equivalent expected CIC. In addition, we may fail to recognize choice as rational if Xt-1 contains private information available to the decision-maker that we do not observe. In general it will be difficult to identify which of these conditions is responsible for rationality failures.
This utility formulation leaves out the possibility that AOOP cost risk is correlated with other risks entering w so that they must be evaluated jointly. It ignores plan attributes such as service quality and pharmacy store convenience that may enter preferences. It also treats rational plan choice as a one-year decision that does not require intertemporal optimization of a discounted stream of utilities. Since the consumer is free to re-optimize in each annual open enrollment period, the conditions for separability of the intertemporal problem into a series of one-year decisions are largely met. However, factors that could reintroduce an intertemporal element would be switching costs between plans, leading consumers to prefer plans with good expected long-term performance even if they are not first-year optimal, or intertemporally non-separable preferences in which habit and inertia enter the determination of consumer well-being.
To test our expected utility formulation, we estimate multinomial logit models of plan choice with the certainty-equivalent expected CIC components as explanatory variables: Premium, MAOOP, ECIC = Premium + MAOOP, and VAOOP. The results are given in Table 5 for each of the plan years 2007 and 2008, for two different constructions of the expectations MAOOP and VAOOP, the first based on Plan Finder, and the second based on our approximation to rational expectations. The details of these expectations constructions are given in Section 4.2. We are unable to include in these models measures of plan quality, such as telephone response and appeal resolution times. These are reported in CMS public data on plans, but are not provided for the encrypted plans in our sample of Part D claims. We do include dummy variables to capture brand and quality differences that vary by insurer; these have little effect on the coefficients of the variables of interest. In Table 5, models (1) for 2007 and (4) for 2008 correspond to the assumptions that people are risk neutral and that they weigh premiums and expected out-of-pocket prescription costs the same. In both years and with both expectations models, the effect of expected CIC is highly significant and of expected sign, but the share of likelihood explained by the models (pseudo R2) is low and a $10 per month difference in expected CIC between plans would induce only about 56% of people to choose the less expensive plan. Thus, sensitivity to cost appears to be low, and choices that fail to achieve minimum expected cost are common. The rational expectations construction explains choice a little better than the Plan Finder construction. The larger magnitude of the coefficient on MAOOP in the rational construction is consistent with the hypothesis that the Plan Finder construction less accurately approximates the quantity that drives choice.
Table 5.
Multinomial logit models predicting Medicare Part D plan choice in 2007 and 2008
| 2007
|
2008
|
|||||
|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | |
| Static (Plan finder) decision rule | ||||||
|
| ||||||
| ECIC | −1.891E-03 | −1.024E-03 | ||||
| 4.550E-06 | 3.560E-06 | |||||
| Premium | −2.140E-03 | -2.140E-03 | −1.370E-03 | −1.537E-03 | ||
| 6.060E-06 | 6.010E-06 | 4.590E-06 | 4.800E-06 | |||
| MAOOP | −1.620E-03 | −1.617E-03 | −4.471E-04 | −1.172E-03 | ||
| 6.130E-06 | 6.170E-06 | 5.570E-06 | 6.630E-06 | |||
| VAOOP | −1.310E-08 | 3.810E-06 | ||||
| 2.650E-09 | 1.890E-08 | |||||
|
| ||||||
| Pseudo R2 | 3.185E-01 | 3.191E-01 | 3.191E-01 | 2.692E-01 | 2.711E-01 | 2.749E-01 |
| α | 2.746E-05 | −6.503E-03 | ||||
|
| ||||||
| Rational decision rule | ||||||
|
| ||||||
| ECIC | −2.398E-03 | −1.760E-03 | ||||
| 5.620E-06 | 4.580E-06 | |||||
| Premium | −2.500E-03 | −2.502E-03 | −1.789E-03 | −2.036E-03 | ||
| 6.490E-06 | 6.490E-06 | 4.950E-06 | 5.270E-06 | |||
| MAOOP | −2.235E-03 | −2.229E-03 | −1.679E-03 | −2.108E-03 | ||
| 7.440E-06 | 7.450E-06 | 6.700E-06 | 6.930E-06 | |||
| VAOOP | −7.000E-08 | 3.380E-06 | ||||
| 2.870E-08 | 1.610E-08 | |||||
|
| ||||||
| Pseudo R2 | 3.209E-01 | 3.211E-01 | 3.211E-01 | 2.766E-01 | 2.767E-01 | 2.809E-01 |
| α | 6.280E-05 | −3.206E-03 | ||||
Notes: Standard errors in shaded rows. ECIC: expected value of consumer inclusive cost (the sum of premium and MAOOP); MAOOP: conditional mean of out-of-pocket cost; VAOOP: conditional variance of out-of-pocket cost. The models also include 80 dummy variables for the insurers; these coefficients are not reported. The decision rules are described in Section 4.2.
Models (2) and (4) reported in Table 5 allow different weightings for premiums and expected out-of-pocket prescription costs. With the rational decision rule, the two components have nearly the same weight, with slightly more sensitivity to Premium than to MAOOP. With the Plan Finder decision rule, the difference is pronounced. Thus, there is evidence that “a dollar is not a dollar” for these two cost components. These weightings are consistent with a widely observed behavioral phenomenon that people discount future and ambiguous costs relative to certain cost commitments made in the present. These results are also consistent with the finding by Abaluck and Gruber (2011) that plan choices in Medicare Part D react more strongly to plan premiums than to other relevant features such as expected out-of-pocket cost.
Models (3) and (6) reported in Table 5 include VAOOP, and can be used to estimate the risk-aversion parameter α. The two years give conflicting results, with 2007 showing modest risk aversion18, and 2008 showing risk preference. We compared the expected utility of a standard (silver) plan with that of a gold plan with generic gap coverage, and find in 2007 that the average annual premium difference that makes a risk-neutral person indifferent between the two is $100.38, and that which makes a person with the risk aversion parameter estimated with the rational decision rule in Model (3) indifferent between the two is $96.25.19 Thus, risk aversion in 2007 is not economically significant. We leave for future research the mystery of apparent risk preference in 2008, but note that the risk associated with Part D plans is quite difficult to determine, and consumers may be responding to plan features correlated with actual risk rather than to the risk itself.
We conclude from our preliminary analysis that expected CIC is a reasonable criterion for assessing the quality of plan choice, that a source of failures to minimize expected CIC may be that consumers place a higher weight on premiums than on MAOOP, and that risk preferences are not a consistent source of failures to minimize expected CIC.
4.2 Ex post and ex ante optimization errors
We take an ex ante approach to evaluation of the quality of Part D plan choices, comparing the expected performance of actual choices and various benchmark decision rules, conditioned on the information that people had access to at the time of choice. Based on our preliminary analysis in section 4.1 of the elements of rational choice and apparent behavioral determinants of choice, we take as metrics of performance relative to each benchmark the averages over our working sample of the conditional probabilities that actual choice had higher expected consumer inclusive cost (ECIC), and of the conditional expected excess cost, the difference in ECIC between these decision rules. Our finding of no consistent, significant risk aversion is the justification for our use of ECIC rather than a certainty-equivalent ECIC that would come more generally out of our CARA utility function framework. The probability of higher ECIC from actual choice than from a benchmark gives information on frequency of failures to attain the benchmark rule performance, while expected excess cost gives information on the economic significance of these failures.
Let CICat denote realized consumer inclusive cost over year t from the actual plan choice made at the end of year t-1 (“plan a”), and CICbt realized consumer inclusive cost over the same year from a benchmark choice (“plan b”). We reconstruct these quantities without or with therapeutic substitution of generic drugs for uncovered branded drugs. The realized excess expenditure CICat – CICbt is an ex post measure of regret with respect to benchmark plan b experienced by the individual. This quantity may influence the person’s decision-making attention and focus at the end of year t for its plan choice in year t+1, but unless the individual has perfect foresight, it is unknown at the end of year t-1 when a plan is chosen for year t. Regret may be wistful (“If I had known then what I know now …”) or feasible (“If I had only checked Plan Finder …”); the difference is whether benchmark rule b could have been implemented with information available at the time of choice. Regret can be averaged over the working sample and interpreted as an ex post measure of the quality of plan choice relative to a wistful or feasible benchmark.
Our ex ante performance measures are designed to depend only on information available at the time a plan is chosen, so that against a feasible benchmark rule, they provide a practical assessment of opportunities missed. They start from the probability of overspending, and expected overspending, conditioned on the information Xt-1 available to an individual at the time of plan t choice. These quantities are then averaged over the information sets in the population:
The final equalities, showing that these measures are simply unconditional expectations over the population, are applications of the law of iterated expectations. We estimate these expectations by replacing them with their empirical equivalents, averages over the working sample.20
With these definitions, we can clarify the relationship between the ex ante performance measures for consumer decision making that we adopt, and an ex post measure employed previously by Ketcham et al. (2012) and emphasized by Zhang and Zhou (2012) that calculates average regret relative to the benchmark of perfect foresight. The distinction between ex ante and ex post performance metrics comes from whether or not the benchmark is feasible, since for a given benchmark the estimate of expected excess spending in the ex ante calculation is identical to the average of regret in the ex post calculation. The ex post comparison with the perfect foresight rule is potentially quite misleading, as even a consumer who makes a fully rational expected-utility maximizing choice can have large wistful regret relative to the perfect foresight benchmark when uncertainty is high. However, differences in average ex post excess spending, relative to the perfect foresight benchmark, between actual choice and a feasible benchmark choice will coincide with ex ante average excess spending. (This result depends on linearity, and does not hold for calculations of shares with excess spending.)
To implement our performance calculations, we consider the (infeasible) perfect foresight benchmark, and four feasible benchmarks: the CMS Plan Finder rule that picks out the plan with the lowest CIC given the previous year’s medicine cabinet, a random benchmark that picks from available plans at random, a minimum premium benchmark that selects a plans that has the lowest annual premium, and a “rational” benchmark that picks a plan to minimize ECIC, conditioned on information available on health and drug needs at the time of plan choice. We implement these benchmark rules as follows:21
Perfect foresight: In year t-1, the consumer forecasts exactly her drug needs in year t. This rule is infeasible except under unrealistic information conditions, but it is an ingredient in ex post performance measures and should give an upper bound for expected excess spending.
Plan Finder: The consumer chooses the plan that minimizes CIC, given the realized drug cabinet in year t-1. In effect, drug use in year t is expected to be the same as in year t-1. Let CICSkt = FBD(MCt-1, k, t) denote this static expected CIC, and call it the Plan Finder Predicted CIC (PFPCIC). Brand and generic substitutions are made when the CIC simulations are made with therapeutic substitution. Actual choice should have zero excess spending when consumers follow the recommendation of the CMS Plan Finder, except for differences in AOOP costs resulting from pharmacy choices, and differences between actual and simulated empirical formularies (see Appendix C).
Random: A plan is drawn at random from those available, in effect all plans are treated as if they have the same expected CIC.
Minimum premium: A related decision rule prescribes that a plan is selected at random from those with the smallest premium, in effect all plans are treated as if they have the same expected AOOP costs, insofar as this is considered at all.
“Rational”: We assume that consumers form statistically realistic forecasts of MAOOP(k,Xt-1) for relevant information Xt-1, and that they choose a plan k to minimize ECIC(k,Xt-1) = Premiumk + MAOOP(k,Xt-1).22
The random and minimum premium benchmarks are simple heuristics that should be in the information sets of even minimally informed consumers.23 The “rational” model is the ultimate benchmark for “good” consumer decision-making in our analysis, but is also the most complex and computationally burdensome rule in that it requires specification of the year t-1 information available to and considered by the individual, and calculation of the conditional forecast MAOOP(k,Xt-1). Because of the high dimensionality of Xt-1 when it includes MCt-1, it is impractical to estimate nonparametrically the conditional density f(MCt | k,Xt-1) and use this to estimate directly the moment MAOOP(k,Xt-1). Instead, we use a “method of sieves” and a one-dimensional “sufficient statistic” for the impact of last year’s medicine cabinet to estimate this moment semiparametrically. Our method does not take advantage of the known fine structure of the nonlinear mapping CICt = FBD(MCtk, t), but with sufficient flexibility in the sieve specification we can in principle recover the relevant aspects of this structure from the data. To implement this procedure, we assume first that the static expectation CICSkt = FBD(MCt-1, k,t) is a sufficient statistic for the information conveyed by a consumer’s prior medicine cabinet MCt-1, and second that CICSkt and a limited number of other demographic and health characteristics in Xt-1 influence the conditional density of MCt through a single linear index H(k,Xt-1)βkt, where H is a vector of transformations of Xt-1 and βkt is a vector of parameters for each plan and year. This implementation is also consistent with an “adaptive expectations” model in which consumers start with the static expectation and adjust toward the average CIC for consumers with similar demographic and health attributes.
The rational decision rule requires specification of the set of variables on which expectations are conditioned. We are obviously limited by the scope of available data. In Heiss et al. (2012), we compare various specifications; here we consider only one specification that includes all available variables in Xt-1. These variables and transformations are defined as follows:
PFPCIC (Plan Finder predicted CIC), i.e., the static expectation CICSkt = FBD(MCt-1,k,t)
A linear spline in age
Gender
CCW, a vector of indicator functions of 23 chronic conditions derived from the Chronic Conditions Warehouse (CCW); see Appendix A. The CCW employs algorithms based on International Classification of Disease Version 9 (ICD-9) codes, the type of claim (inpatient, outpatient, physician services, etc.), and the number of claims within a given reference time period.24
CCW score, equivalent to the Hierarchical Condition Category (HCC) risk score calculated by Center for Medicare and Medicaid Services (CMS). The HCC risk score is a measure of expected drug costs based on diagnosis codes and demographic factors and is used by CMS to adjust Medicare Parts C and D payments to insurance plans.25
We assume that this “rational” consumer has ECIC(k,Xt-1) obtained by summing Premiumk and the best linear prediction obtained from a regression over the working sample of realized CICkt on the variables above, and selects a plan to minimize this ECIC.
Our primary measures of the quality of the consumer’s plan choice are population averages of the ex ante probabilities of expected excess spending and of the levels of expected excess spending, relative to each of the five benchmarks we consider. For the perfect foresight benchmark, this reduces to the average ex post wistful regret from failing to choose the plan that is ex post optimal. For the remaining benchmarks, all of which are arguably feasible, this gives population averages of excess spending probabilities and levels relative to decision rules the
4.3 Results
All simulations reported in this section are conducted for the entire working samples of 2007 and 2008 (data from 2006 is used to construct the variables that are the information for decisions made at the end of 2006 for plan year 2007). Due to the large samples, statistical sampling errors are negligible, and are not reported. Following our discussion in the previous section, the analysis takes an ex ante perspective to evaluate plan choices, with population averages of conditional expectations of our performance comparisons of actual and benchmark rule choices given prior year information estimated by unconditional empirical expectations of ex post realizations of these comparisons. For feasible benchmarks such as random, minimum premium, Plan Finder, and “rational”, these estimate the ex ante probability of excess spending and the expected level of excess spending. For the wistful benchmark of perfect foresight, this calculation reduces to ex post comparisons of the form that have appeared previously in the literature.
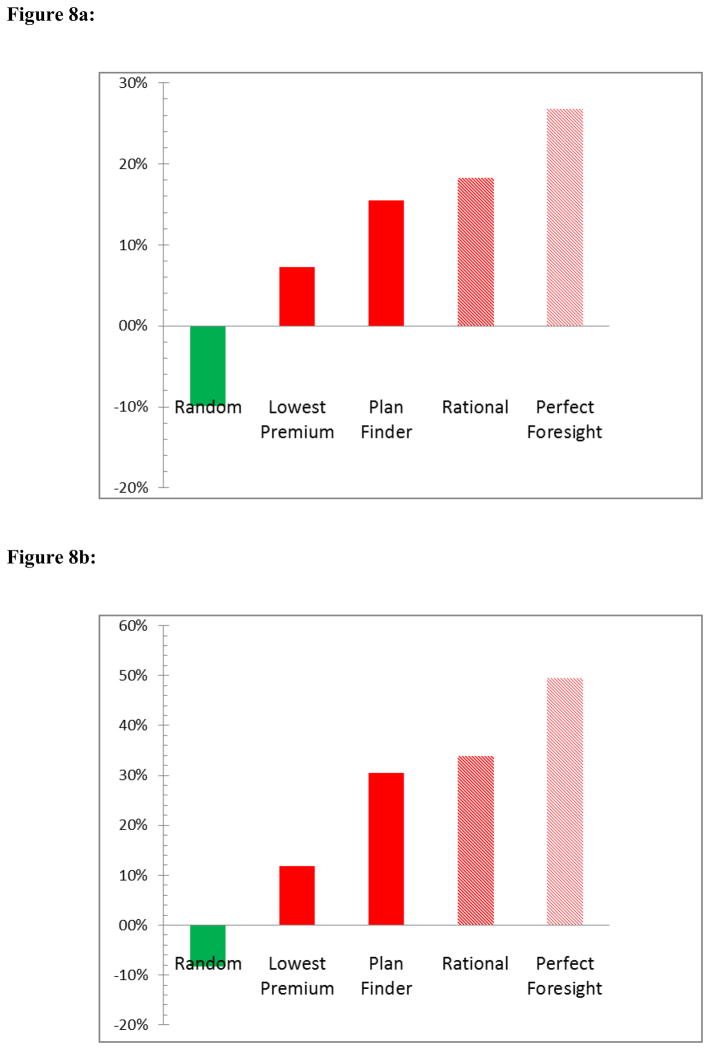
Table 6 reports our estimates of the population proportions of individuals whose actual plan choice is worse in terms of expected consumer inclusive cost than each of the benchmarks we consider, and our estimates of average overspending in (current) dollars per year. The higher these numbers, the worse consumers are doing relative to each benchmark. The top panel contains results for 2007 and 2008 plans without therapeutic substitution; the bottom panel for 2008 introduces therapeutic substitution. First, we observe in the case of no therapeutic substitution that the plans that individuals chose for 2007 and 2008 were rarely as good as Plan Finder, which itself is expected to be inferior to a “rational” plan that takes account of the probability of developing new health conditions that require additional drugs: In 2007, 78% of consumers would have been better off choosing the plan suggested by the Plan Finder; in 2008, this proportion was 75.5%. Average overspending compared to the plan suggested by Plan Finder was about $200 per year in both 2007 and 2008. Following the rational decision rule we described above results in very similar numbers.26 A simple feasible decision rule – choosing the cheapest plan – would still allow about two thirds of consumers to end up with lower total spending than in their actual choice. Perhaps even more surprisingly, about one quarter of consumers do worse than if they had chosen a plan at random; however, on average consumers would spend more with randomly chosen plans than with their actual choices, as indicated by the negative average overspending dollar amounts.
Table 6.
Actual plan choices vs. optimal choices implied by alternative decision rules
| 2007 and 2008; no therapeutic substitution
| ||||
|---|---|---|---|---|
| Share with higher spending
|
Avg. overspending
|
|||
| 2007 | 2008 | 2007 | 2008 | |
| Plan Finder | 78.0% | 75.5% | 197.91 | 195.68 |
| Random | 23.7% | 22.5% | −96.47 | −124.61 |
| Lowest premium | 66.0% | 65.1% | 115.52 | 91.25 |
| Rational | 77.4% | 76.7% | 196.06 | 230.57 |
| Perfect foresight | 93.7% | 89.1% | 315.05 | 338.53 |
| 2008; no therapeutic substitution vs. generic therapeutic substitution
| ||||
|---|---|---|---|---|
| Share with higher spending
|
Avg. overspending
|
|||
| No subst. | Subst. | No subst. | Subst. | |
| Plan Finder | 75.5% | 80.3% | 195.68 | 313.51 |
| Random | 22.5% | 27.5% | −124.61 | −85.95 |
| Lowest premium | 65.1% | 61.6% | 91.25 | 121.04 |
| Rational | 76.7% | 80.6% | 230.57 | 349.31 |
| Perfect foresight | 89.1% | 91.6% | 338.53 | 509.70 |
Notes: The table shows the share of chosen plans that resulted in higher spending than what would have been achieved with the plan choice implied by a bechmark decision rule; average of excess spending (in current dollars) implied by the chosen plan compared to the benchmark plan.
Our results for the perfect foresight rule, a benchmark that compares actual choice with the ex post optimum, indicate that 6.3% of consumers in 2007 achieve this optimum, while the remainder do worse; the proportion that were ex post optimal was slightly higher in 2008. Average ex post overspending was more than $300 dollars per year in 2007 compared to the perfect foresight benchmark.
Taking account therapeutic substitution, these numbers change slightly, as reported in the bottom panel of Table 6. In general, alternatives to a chosen plan will have lower cost if substitutions are assumed, but there can be exceptions when a drug is in the alternative plan formulary, but on a higher tier than in the chosen plan formulary, or when a drug in the chosen plan is much more expensive than the least cost therapeutic equivalent that is substituted in this plan.
Figures 8a and 8b compare the magnitudes of the decision errors across the different benchmark decision rules, expressed as a percentage of the CIC of the chosen plan, averaged over all observed plan choices in our working sample. Figure 8a reports these numbers for the case without therapeutic substitution; Figure 8b compares the cases without and with therapeutic substitution in 2008. With therapeutic substitution, consumers lost 30.5% relative to the Plan Finder decision rule, compared with 15.5% without therapeutic substitution. Both choices would have been attainable at (arguably) small decision cost. The average percentage losses are larger when the benchmarks are given by the rational or perfect foresight rules, but these benchmarks are more difficult or impossible to attain. Choosing a random plan would have made consumers worse off, on average, as noted above.
Figure 8.
Figure 8a. Reduction in CIC associated with optimal choices implied by benchmark decision rules, relative to the chosen plan’s CIC (2008, no substitution)
Figure 8b. Reduction in CIC associated with optimal choices implied by benchmark decision rules, relative to the chosen plan’s CIC (2008, with therapeutic substitution)
It is useful to compare our results with Zhou and Zang (2012), who examine similar questions of plan choice using a 5 percent sample of 2009 Part D claims data. By comparing actual choices with those that minimize the estimated spending of each beneficiary if enrolled in each of the plans in her choice set, their simulation is similar to use of the perfect foresight benchmark, giving an ex post analysis. They do analyze the case in which beneficiaries predict drug use based on prior year claims (akin to our Plan Finder simulations), but only as a sensitivity test. The overspending amounts they compute are of the same orders of magnitude as ours. They argue that consumer decision making is likely to fall between perfect prediction and prior year history, whereas we focus on the results of following Plan Finder as well as the very simple random choice and minimum premium heuristics. As we argue above, we believe that the case of perfect foresight, which is also the focus of the study by Ketcham et al. (2012), should only be viewed as a wistful benchmark that does not provide a realistic measure of the losses associated with poor health-plan choices relative to those achievable using feasible decision rules.
Finally, we consider whether consumers’ choices improve over time, as claimed in the study of Ketcham et al. (2012). For this purpose, we concentrated on 2008 plan choices but stratify the sample according to whether a consumer first enrolled in a Medicare Part D stand-alone plan in 2008 (11%), stayed in her 2007 plan (79%), or switched to a different plan from 2007 to 2008 (10%). The results are displayed in Table 7. We observe that the fraction of consumers who chose plans that are ex ante worse than the one suggested by plan finder is in fact smaller among the switchers (about 56%) than among the stayers (about 80%). Thus, plan choices improved for the switchers, but still there is a considerable fraction of individuals who overspend in this analysis. Interestingly, the newly enrolled do better than the stayers but not as well as the switchers. Similar relative results hold when ex ante optimal plans are determined using the random, lowest premium, rational, and perfect foresight rules.
Table 7.
Actual plan choices vs. optimal choices implied by alternative decision rules by switching status in 2008
| Total | New | Stayed | Switched | |
|---|---|---|---|---|
|
| ||||
| Share of plans in sample | ||||
|
| ||||
| 100.0% | 10.9% | 78.8% | 10.3% | |
|
| ||||
| Share with higher spending | ||||
| Plan Finder | 75.5% | 67.3% | 79.2% | 55.8% |
| Random | 22.5% | 19.0% | 24.5% | 10.7% |
| Lowest premium | 65.1% | 60.9% | 68.1% | 46.5% |
| Rational | 76.7% | 69.8% | 80.3% | 56.7% |
| Perfect foresight | 89.1% | 86.8% | 91.1% | 75.6% |
Notes: The table shows the share of chosen plans that resulted in higher spending than what would have been achieved with the plan choice implied by a benchmark decision rule. The columns stratify the sample by whether a consumer chose her first plan in 2008 (“new”), the same plan as in 2007 was chosen (“stayed”), or a different plan than in 2007 was chosen (“switched”).
To summarize, even simple decision rules such as “choose the lowest premium plan” or “choose the plan suggested by Plan Finder” would have generated considerably lower ex ante expected spending. More sophisticated rules, such as our version of a rational decision rule that conditions on a small set of observable end-of-prior-year health and drug use characteristics, would have brought even larger savings. In line with Ketcham et al. (2012), we observe some improvement of plan choices for switchers, but even among them overspending is still considerable. It is hard to reconcile these monetary losses with the implicit decision costs associated with using a tool such as Plan Finder. In fact, Medicare might take a lesson from Geico’s popular advertisement for its car insurance: “15 minutes with Plan Finder could save you 15 percent or more on your prescription drugs.”
We cautioned earlier that our performance measures may overstate deviations from expected utility maximization if individuals place large values on plan features other than those we consider in our analysis, such as convenience and customer service, if they implement an intertemporal optimization strategy (rather than decide only for the coming year) due to very high switching costs, or if they value risk quite differently than we can account for using the CARA utility function framework. However, it would be surprising if these factors were sufficiently large for a genuinely rational consumer to produce the levels of excess spending that we estimate. We believe that it is difficult to avoid the conclusion that the acuity of consumer choice among Part D plans is low.
5. Conclusions
This paper shows that there is potentially great scientific benefit in using the detailed information on health, drug use, Part D plan choice, premiums, and OOP costs available in the Medicare A, B, D claims data. To deal with the inability to link encrypted plan information in the Part D claims data to CMS public files on plan characteristics, we have constructed empirical formularies and benefit designs using data from the sub-sample of individuals enrolled in each plan. This effort is successful in reproducing the OOP costs of chosen plans, and appears to be valid for calculating the CIC for both chosen and alternative plans. While the claims data we used in this paper provide reliable information on plan choices and allow precise estimates of the costs of alternative plans, the lack of beneficiary characteristics prevents us from studying the determinants of choice quality, so we leave this important issue to further research.
Our analysis of enrollment and choice between levels of plan generosity suggests that the share of eligible consumers without drug insurance is in the range one would expect if risk reduction and the option value of avoiding late enrollment penalties in the future are ignored and the only criterion is whether enrollment is first-year actuarially favorable. In choice between Silver (e.g., standard) and Gold (e.g., generic gap coverage) plans, the evidence is that consumers are undersubscribing to Gold plans. This result is consistent with the finding by others (inter alia, Abaluck and Gruber, 2011, for Medicare Part D, and Ericson and Starc, 2012, for the Massachusetts Connector) that consumers pay more attention to premiums than to benefit generosity, so that they are nudged toward low-premium standard or equivalent plans.
Calculations of ex ante excess spending relative to feasible benchmark decision rules, without and with therapeutic substitution, and of the optimal choices under various expectations models and decision rules for each individual, suggest that fewer than 25 percent of individuals enroll in plans that are ex ante as good as the least cost plan specified by the CMS Plan Finder benchmark that conditions next year’s plan choice only on the drugs consumed in the current year. Relative to this benchmark, enrollees lost on average about $300 per year. While these losses are rather modest when compared to the losses associated with not enrolling at all, an issue we have studied extensively in earlier research, they are difficult to reconcile with decision costs alone. It appears that a sizeable fraction of consumers do not optimize effectively, and specifically fail in a large majority of cases to do as well as they would if they adopted the readily available CMS Plan Finder decision rule. At least for Medicare Part D plans with their rather complex structure, our results do not support the proposition that consumers can make and benefit from good choices in private health insurance markets, and “vote with their feet” to direct health care resources to their best use.
Recent behavioral research on decision-making of older individuals suggests some reasons for why plan choices in Medicare Part D are far from optimal. In a recent study of plan choice in Massachusetts Health Connector, Sinaiko et al. (2013) stress that prescription plan choices need to be simplified, and that consumers would benefit from guidance by decision-support tools. One important aspect is the number of options: In controlled laboratory experiments with subjects covering a wide age range, Besedeš et al. (2012a, 2012b) show that the probability of selecting the optimal plan declines as the number of options increases.27 Perhaps not surprisingly but nevertheless important in the context of health plan choice, the decline of choice quality with the size of the choice set is more pronounced for older subjects.28 Besedeš et al. relate this finding to an increased tendency to use simplifying heuristics among older individuals. The oldest subjects are also more likely to make entirely random choices. Agarwal et al. (2009) show that in the context of credit transactions, decision making first improves and then deteriorates with age, with cost-minimizing decisions being made by individuals in their early 50s. They relate these findings, quite plausibly, to a decline in cognitive ability.
Findings such as these support calls for a reduction of the number of available options (e.g., Perry et al., 2006; Handel and Kolstad, 2013). We believe this could be done without reducing plan competition or substantially restricting consumer welfare gains by focusing competition on premiums for standardized formulary and benefit designs; Lucarelli et al. (2012) give a contrary argument. Further, it may be possible to develop decision aids that help consumers in structuring and solving the choice problem, as in the experimental analysis of alternative “choice architectures” in health-insurance choice by Ericson et al.(2012). Reminding consumers during open enrollment periods that they have a plan choice, pointing out the availability and features of CMS Plan Finder, and nudging consumers by revealing their excess spending in their previous plan, might all help improve performance and bring the Part D market closer to the competitive ideal.
Acknowledgments
This research was supported by the Behavioral and Social Research program of the National Institute on Aging (grants P01AG033559 and RC4AG039036), with additional support from the E. Morris Cox Fund at the University of California, Berkeley. We thank Mark Duggan, Amy Finkelstein, Amelie Wuppermann, the referees, guest editor Joseph Newhouse, and audiences at the Universities of Augsburg, Duisburg, Hamburg, Mannheim, and Texas-Austin, at Tilburg University, at the 2012 annual meetings of the American Economic Association in Chicago and the Verein für Socialpolitik in Göttingen for helpful comments and discussions. We thank Patricia St. Clair, Dana Goldman, and Armando Franco for their support of the data construction effort and the coding of health conditions; and Sarah Axeen for additional assistance. Heiss and Winter thank the Schaeffer Health Policy Center at USC, and Winter also thanks the Center for Health and Wellbeing at Princeton University, for their hospitality.
Appendix A. Data sources and working samples
The claims records available for this study are those from the Medicare denominator files (lists of all persons in the United States who qualify for Medicare benefits) for the years 2006, 2007, and 2008 with a Health Insurance Claim (HIC) code ending in the digits ‘0’ or ‘5’, which corresponds to a 20 percent draw. When an individual moves from one household to another, and is not a primary beneficiary in either, the HIC code assigned to this beneficiary switches to reflect the new primary beneficiary, and will no longer necessarily end in ‘0’ or ‘5’. However, CMS states that as of 2006, these individuals are tracked through the HIC code change, and their claims are retained under their original HIC code in the 20 percent draw. It is unclear how claims records prior to 2006 are handled for HIC code switchers.
From the 20 percent draw, we extract working samples of 1,139,469 individuals in 2006, 1,249,301 in 2007, and 1,307,396 in 2008 from our target population of enrollees aged 65+ who had sufficient data on plan alternatives and drug use to compare premiums and copayments in their chosen plan with alternatives not chosen. Effectively, this restricts the working samples to Medicare enrollees on stand-alone PDP who do not have retiree or low-income subsidy (LIS) status that limits their alternatives and/or changes their cost incentives, and eliminates people with incomplete annual Part D claims records. Table A1 lists the successive screens used to obtain these working samples, and gives the number of individuals remaining after each exclusion step, together with average age, shares of females and of whites, and the average number of chronic health conditions.
The claims records do not contain information on income (other than low-income status), wealth, education, or cognition measures that would allow a more complete description of selection in our working samples.29 Enrollees in stand-alone PDP plans are slightly older on average than U.S. residents aged 65+, with larger shares of females and non-whites. The remaining exclusion criteria select working samples that are slightly younger than the sample of stand-alone PDP enrollees, with reduced female and non-white shares, primarily because the dual-eligible and LIS classification has higher participation from females and minorities.
Table A2 gives a breakdown by age of the patterns of part and full-year Medicare enrollment. Most enrollees enter upon reaching age 65, with about 2% initially enrolling after age 65. Nearly 25% of the enrollees that begin the year at age 64 were enrolled throughout the year, indicating that they are dual eligibles enrolled due to a severe chronic disability. The proportion of leavers due to death is in the range of 1 to 3%, reflecting the general population death rate for this decade.
Table A3 lists 23 health conditions we consider. An indicator is one in the plan year 2006 (resp., 2007, 2008) if the person “ever had” the corresponding condition, as diagnosed from their observed claims records starting from 2002 or initial enrollment in Medicare A/B, whichever came later, up through December 31, 2005 (resp., 2006, 2007). Thus, the medical event history used to determine these health conditions would have been known to each individual at the time of their 2006 (resp., 2007, 2008) Part D plan choice. The table reports the prevalence in our working samples of these ever-had indicators in each plan year.
Our indicators are obtained using definitions and coding from the Chronic Condition Warehouse (CCW), a CMS-sponsored effort to identify chronic health conditions and risk factors.30 CCW obtains “currently-has” indicators based on valid ICD-9, CPT4, and HCPSC diagnostic codes from Part A/B claim records over reference time periods of one to three years up to the calculation date, as indicated in Table A3. An “ever had” condition indicator is one if any of the corresponding CCW “currently-has” indicators is one over the person’s observed claim history preceding the plan year. We extend the CCW classification of conditions used during the 2006 to 2008 period by adding hypertension and HIV/AIDS as conditions.31
The qualifying conditions for the CCW indicators (inpatient, hospital out-patient (HOP), and carrier claims) have been established on the basis of medical studies to identify most clinical conditions while minimizing false positives from rule-out diagnoses. Enrollees without full claims records over a reference period, such as Medicare Advantage and new enrollees, are coded on the basis of the claims they do have, and may be undercounted for conditions. On the other hand, our “ever-had” indices will overstate conditions requiring treatment if the person has been cured or is in full remission.
As Table A3 shows, there is an increase in the prevalence of each “ever-had” health condition each year, and correspondingly an increase in mean numbers of health conditions reported in Table A1. These trends resemble but are stronger than those calculated by CCW over the period 2000–2009 in 5 percent samples of the full Medicare population.32 These trends are not easily explained by the relatively minor changes in the composition of the population or of our working samples from year to year, leaving the possibilities that that they are driven by trends in the clinical health of the population or trends in diagnostic practice. We leave this puzzle for future research.
A key concept in our analysis to the total drug bill (TDB), defined as the annual sum of pharmacy prices in the chosen stand-alone Part D plan an individual’s prescriptions (her “medicine cabinet”), as reported in her Part D claims files. Table A4 contains the results of linear regressions of TDB on the indicators for “ever-had” chronic health conditions at the end of the years preceding each of the years 2006, 2007, and 2008 using our working samples. The coefficient on each condition is an estimate of the expected cost of drug therapy in a year, given health indicators based on claims in the preceding three years. Analogous regressions with log TDB as the dependent variable have coefficients interpreted as expected percentage increases in TDB due to each condition. The highest costs are associated with Alzheimer’s Disease and related disorders, followed by diabetes, depression, HIV/AIDS, breast cancer, chronic kidney disease, and chronic obstructive pulmonary disease. The largest year-over-year changes are the increase in the cost of Alzheimers related treatments, and decreases in the costs of treating osteoporosis, hypertension, and acute myocardial infarction. The small or negative coefficients for treatment of cancers, and for hip fractures, reflect sample selection, as persons with lower comorbidity rates (and lower TDB) and consequently higher rates of cures or remissions remain in the sample through the year, while persons with high comorbidity rates have higher mortality rates within the year that remove them from the sample.
Table A4.
Regressions of total drug bills on chronic health indicators
| Chronic Condition | TDB ($US, current year) | Logs(TDB) | ||||||
|---|---|---|---|---|---|---|---|---|
| 2007 | 2008 | 2007 | 2008 | |||||
| Acute Myocardial Infarction | 262.9 | (9.95) | 221.9 | (9.60) | 0.125 | (0.00) | 0.114 | (0.00) |
| Alzheimer’s Disease | 716.9 | (13.78) | 810.0 | (13.96) | 0.283 | (0.01) | 0.308 | (0.01) |
| Alzheimer’s Disease, Senile Dementia, Related Disorders | 547.8 | (9.06) | 589.9 | (9.08) | 0.216 | (0.00) | 0.230 | (0.00) |
| Atrial Fibrillation | 21.7 | (4.96) | 10.8 | (4.99) | 0.044 | (0.00) | 0.040 | (0.00) |
| Breast Cancer | 380.3 | (8.19) | 389.7 | (8.16) | 0.188 | (0.00) | 0.188 | (0.00) |
| Cataract | −45.1 | (3.16) | −50.2 | (3.24) | −0.005 | (0.00) | −0.010 | (0.00) |
| Chronic Kidney Disease | 300.4 | (5.55) | 288.3 | (5.28) | 0.138 | (0.00) | 0.135 | (0.00) |
| Chronic Obstructive Pulmonary Disease | 285.0 | (4.56) | 310.5 | (4.55) | 0.149 | (0.00) | 0.163 | (0.00) |
| Colorectal Cancer | −184.9 | (10.37) | −179.4 | (10.40) | −0.102 | (0.01) | −0.095 | (0.01) |
| Depression | 410.9 | (4.51) | 406.5 | (4.43) | 0.201 | (0.00) | 0.200 | (0.00) |
| Diabetes | 480.1 | (3.66) | 485.5 | (3.65) | 0.264 | (0.00) | 0.269 | (0.00) |
| Endometrial Cancer | −66.4 | (22.66) | −66.6 | (21.92) | −0.043 | (0.01) | −0.047 | (0.01) |
| Glaucoma | 144.8 | (3.91) | 159.7 | (3.91) | 0.112 | (0.00) | 0.120 | (0.00) |
| Heart Failure | 193.6 | (4.65) | 150.6 | (4.67) | 0.086 | (0.00) | 0.062 | (0.00) |
| Hip/Pelvic Fracture | 8.2 | (11.96) | −14.1 | (11.63) | −0.034 | (0.01) | −0.039 | (0.01) |
| HIV/AIDS | 351.6 | (109.59) | 505.8 | (126.13) | 0.130 | (0.05) | 0.193 | (0.05) |
| Hypertension | 70.4 | (4.02) | 53.2 | (4.12) | 0.154 | (0.00) | 0.142 | (0.00) |
| Ischemic Heart Disease | 294.2 | (3.42) | 291.4 | (3.46) | 0.199 | (0.00) | 0.202 | (0.00) |
| Lung Cancer | 83.3 | (17.62) | 112.6 | (17.44) | 0.047 | (0.01) | 0.055 | (0.01) |
| Osteoporosis | 196.9 | (3.45) | 148.1 | (3.46) | 0.151 | (0.00) | 0.118 | (0.00) |
| Prostate Cancer | −1.4 | (7.48) | 22.4 | (7.45) | −0.005 | (0.00) | 0.005 | (0.00) |
| Rheumatoid Arthritis/Osteoarthritis | 81.6 | (3.47) | 78.4 | (3.46) | 0.051 | (0.00) | 0.054 | (0.00) |
| Stroke/Transient Ischemic Attack | 265.1 | (5.46) | 253.9 | (5.42) | 0.130 | (0.00) | 0.125 | (0.00) |
| Constant | 1456.2 | (4.54) | 1429.8 | (4.51) | 6.834 | (0.00) | 6.804 | (0.00) |
|
| ||||||||
| R2 | 0.133 | 0.135 | 0.125 | 0.125 | ||||
| N | 1016478 | 1059747 | 1016478 | 1059747 | ||||
Note: Robust standard errors are reported in parentheses.
Appendix B. Empirical formulary construction
In the Part D claims records, there are 124 distinct formulary identifiers in 2007 and 108 in 2008, excluding those offered only in U.S. territories, with some identifiers appearing in multiple plans offered by the same insurer. Due to CMS encryption and data use rules, we cannot associate these formulary identifiers with published formularies of insurers. Therefore, for each formulary identifier in each of the years 2006, 2007, and 2008, we construct an empirical formulary given by the union of all the NDC codes of claims of enrollees with this formulary identifier. The empirical formularies account for NDC codes found in all Part D claims, not just those NDC codes observed in our working samples. This larger set of claims allows us to catch more NDCs on each formulary and is not problematic because individuals excluded from our working sample still face the same choice set of drugs from any given formulary.
The most popular formulary in 2007 had almost 900,000 enrollees, while the median formulary had 7,385 enrollees. The chance that a formulary drug will be captured by this method can be low for uncommon drugs in formularies with modest numbers of enrollees. A drug ranked 500 in prescription frequency is used by about 0.21 percent of enrollees, and in the median enrollment empirical formulary is almost certainly captured; the probability is 1 - (1 - 0.0021)7385. For the drug ranked 1000, the prescription frequency is 0.03 percent, and the chance of capture is 90 percent, while for the drug ranked 2000, the prescription frequency is 0.003 percent and the chance of capture is 19 percent.
Whether to include a specific drug in the empirical formulary is a statistical decision problem. Let y = 1 if the drug is in the true formulary, 0 otherwise. We know the share s of all PDP enrollees who have this drug in their published formulary, the share p of all PDP enrollees who take this drug (whether or not it is in their formulary), and an indicator d =1 if the drug is observed in the claims data, d = 0 otherwise. Denote our decision rule h(s,p,d) = 1 if we include this drug in the empirical formulary, 0 otherwise. Now the prior probability that the drug is in the actual formulary is s. The probability of observing d = 1 given y = 1 is (1 − p/s)N, where N is the number of enrollees in a formulary, and the probability of observing d = 1 given y = 0 is zero. Hence, the posterior probability of y given d is Prob(y=1|d=1) = 1 and Prob(y=0|d=0) = (1 − s)/[1 − s + s(1 − p/s)N]. We either make a type 1 error with a cost c1 by excluding a drug that is truly in; or a type 2 error with a cost c2 by including a drug that is truly out. Obviously h(s,p,1) = 1. The ratio of the expected costs of type 1 and 2 errors when d = 0 is c1 Prob(1|0)/c2 Prob(0|0) = c1s(1 − p/s)N/c2(1 − s). To minimize expected cost, set h(s,p,0) = 1 when . This has the property for d = 0 that when s is near one, a drug is called in unless p is very high, and when s is low, a drug is called out unless p is very low. A difficulty in implementation is specification of c1/c2. The conservative calculation is to make non-chosen plans look no better than they actually are. Then, c2 > 0 and c1 = 0, and the criterion above becomes h(s,p,0) = 0, the rule we follow. An alternative decision rule with c1 > 0 would strengthen our conclusion that the choices of most consumers are sub-optimal.
Many beneficiaries in stand-alone PDP plans have one or more covered claims for drugs listed on a tier that is higher than the highest tier covered by the plan, or on a tier classified as “NA”. The NDC claims for drugs so classified never appear on a regular tier in other claims for enrollees in the plan, indicating that they are in most cases benefits paid as the result of appeals rather than administrative coding errors or off-schedule purchases such as replacements of lost prescriptions. After empirically examining the cost sharing associated with such claims by benefit phase, we assign “off-tier” claims no coverage in the deductible or doughnut hole, 25 percent coinsurance in the pre-ICL phase, and 5 percent coinsurance in the catastrophic phase. Roughly 20 percent of the sample has at least one off-tier claim, so this is a pervasive phenomenon.
Appendix C. Simulation of TrOOP, AOOP, and CIC for alternative plans
Each person in our working sample in each year has a plan choice set determined by her Medicare region (there are 34 such regions). We run each medicine cabinet under consideration through the formulary of each of the plans in the enrollee’s choice set to determine the TrOOP cost. To determine AOOP, we add to TrOOP the full prescription prices of drugs that are not in the plan’s formulary. Finally, we add the plan premium to determine CIC. For these calculations, full prescription prices of drugs are taken from the chosen formulary. There is minor variation in pharmacy drug prices across insurers and formularies, but some of this is due to CMS fuzzing of prices, and using chosen plan prices eliminates artificial cost differences introduced by fuzzing. In most of the simulations, a current year’s medicine cabinet is evaluated either for prospective plan choice in the following year, or for retrospective assessment of the current year plan choice.
Under the no substitution assumption, a given medicine cabinet is run through all available plans without modification, and drugs outside a plan’s formulary is assumed to be purchased at the full prescription price. Under the therapeutic substitution assumption, a drug in the medicine cabinet that appears in the formularies of all available plans is retained without substitution, but if it is not in every available plan’s formulary, then a therapeutically equivalent drug with the minimum copayment or coinsurance amount in each plan is substituted in the medicine cabinet. In the last case, the substitution rule does not distinguish between branded and generic drugs, and is applied to the chosen plan as well as alternative plans. Therapeutic classes are assigned using the classification in RxNorm33, which we match to the NDC codes in our claims. Then, in these cases, the estimation can assign a lower TrOOP to a chosen plan than is observed. This calculation puts both chosen and alternative plans on a level playing field by assuming that optimization among therapeutic equivalents is done in the same way for all plans. However, it incorrectly penalizes a chosen plan if plan choice was made to obtain a specific drug even though it is apparently not therapeutically cost-effective.
When claims straddle two or more benefit phases, we split the claim into parts corresponding to each benefit phase and apply the associated cost sharing for that component. We make four assumptions about prescription drug utilization:
Same order of drug utilization. Individuals follow the same order of drug consumption in each alternative plan as is actually observed in their chosen plan. Any substitution is based only on the type of drug, but not the quantity or timing of utilization.
Sorting claims on the same date. It is common for multiple prescriptions to be filled on the same day. The order in which such claims are processed may be important for calculating a patient’s TrOOP if one of the claims straddles two benefit phases. To achieve replicable simulation results, some sorting rule must be imposed for claims occurring on the same day. We assume that multiple claims on the same day are sorted first based on the benefit phase recorded in the beneficiary’s chosen plan and are then sorted second based on the total cost of the drug (from low to high).
Pharmacy choice to minimize TrOOP cost. In reality, the choice of where to fill prescriptions is likely based on differences in both TrOOP cost and convenience. However, we assume that beneficiaries will always fill prescriptions at pharmacies and in quantities offering the lowest annual TrOOP cost. Our assumption assures comparability in costs across consumers and plans, and to the extent that higher TrOOP costs incurred at local pharmacies or for more frequent refills simply reflect consumers’ valuations of the added convenience, our measure is the correct benchmark for TrOOP cost comparisons.
Zero price elasticity of drug use. We assume that if a consumer uses a drug in their chosen plan, then they will use the same quantity and dosage of this drug, or a therapeutic equivalent, in any alternative plan, irrespective of price differences across plans and drugs.
Appendix D. Abbreviations and acronyms
| AOOP | Annual Out-of-Pocket cost |
| CCT | Catastrophic Coverage Threshold |
| CIC | Consumer Inclusive Cost = Premium + AOOP |
| CMS | Center for Medicare and Medicaid Services |
| ECIC | Expected CIC = Premium + MAOOP |
| EGWP | Employee Group Waiver Plan |
| FBD | Formulary and Benefit Design |
| HCC | Hierarchical Condition Code |
| HIC | Health Insurance Claim code |
| ICD-9 | Index of Clinical Diseases 2009 |
| ICL | Initial Coverage Limit |
| LIS | Low Income Subsidy |
| MA | Medicare Advantage |
| MC | Medicine Cabinet |
| MAOOP | Conditional mean of AOOP |
| NDC | National Drug Code |
| POS | Point of Service |
| TDB | Total Drug Bill |
| TrOOP | True Out-of-Pocket cost |
| USP | Uniform Standard Pharmaceutical classification |
| VAOOP | Conditional variance of AOOP |
Footnotes
In the first year of Medicare Part D, more than 90% of the eligible population obtained prescription drug coverage, either from a Medicare Part D plan or a source with comparable coverage (Heiss, McFadden, and Winter, 2006).
We have pointed out elsewhere (Heiss, McFadden, and Winter, 2009) that variety in available levels of coverage has diminished sharply for individual buyers in the first three years of operation of the Part D market. Offerings of plans with the most comprehensive coverage have collapsed, and plans with intermediate coverage are at risk of a death spiral of rising premiums and falling enrollment, a phenomenon predicted for this market by Pauly and Zeng (2004) as a consequence of adverse selection, and observed in other health insurance markets; see Cutler and Reber (1998). Union and employer-provided retiree plans that are coordinated with Part D, and Medicare Advantage plans that bundle drug coverage with other medical services in an HMO-like setting, are not subject to the same selection pressures, and continue to offer a variety of coverage levels. However, health insurance provided under retiree plans is dropping, and individual policies for prescription drugs will become more important in the future.
Unlike financial insurance markets where informed arbitragers can offset the influence of “noise traders” and achieve efficient (but possibly unstable) resource allocations, health insurance markets offer limited opportunities for efficiency-improving arbitrage. Consequently, niches where “bait and switch” and similar tactics exploit inattentive consumers may survive. Ericson (2012) provides evidence of such firm behavior in the market for Part D plans. Low consumer acuity may blunt adverse selection and make health insurance markets more stable. However, controlling adverse selection through regulation of health insurance products, enrollment mandates, risk adjustment, and premium support should be more efficient than controlling it by baffling consumers.
There is also important recent research that studies plan choice in health insurance markets other than Medicare Part D. For example, the papers by Sinaiko and Hirth (2011) and Handel (2012) show that substantial numbers of consumers choose dominated plans when they had unambiguously better options. Ericson and Starc (2012) stress the role of preference heterogeneity and rules of thumb in health plan choices made in the new Massachusetts Connector. Handel and Kolstad (2013) study plan choice using linked administrative and survey data which allow them to separately identify risk preferences, information frictions, and plan choice hassle costs. They document substantial inertia and information frictions and show that restricting plan choice would improve welfare substantially.
An enrollee is dual-eligible if on Medicaid or a Medicare Savings Plan and 65+ age-eligible for Medicare.
We make an exception to these rules in tabulations and correlations involving 2006 by including people who in 2006 initially enrolled after January 1, but before the end on May 15 of that year’s extended open enrollment period. For these people, we impute their drug bills and claims on an annual basis by assuming that had they been enrolled for a full year, their imputed January claims would have been the same as their actual claims in their first 31 days of actual enrollment, and that their imputed claim rate for the days between February 1 and 31 days after their actual enrollment would have been the same as their actual claim rate over the year starting from 32 days after their actual initial enrollment.
People on Medicare Advantage (MA) plans do not have the same Part A/B claim detail as people on plans with point-of-service (POS) claims, so that accounts of health conditions based on A/B claims are not reliably comparable, and are understated for MA enrollees. However, there is independent evidence that MA enrollees are on average healthier than POS enrollees; see McGuire, Newhouse, and Sinaiko (2011).
Table A4 reports regressions of total drug bills on our health condition indicators. The results, discussed in detail in appendix A, provide some indication of the contribution of each condition to drug need. CMS uses a hierarchical condition code (HCC) for risk adjustment of Part D enrollees; this is based on a statistical technique that accounts for the property that severe health conditions can encompass multiple basic conditions. Comparison of risk adjustments using the HCC and/or our linear model is left for future research.
The pharmacy price of a prescription is the amount negotiated and paid by the insurer (the benefit) and the enrollee (the copayment), including dispensing fees. It is not adjusted for rebates from pharmaceutical companies to insurers or pharmacies.
Our focus on calendar-year expenditures is appropriate for Part D, where consumers face an end-of-year “open enrollment” period for the plan they will be committed to in the coming year, and current year expenditures can be determined from information provided by their current insurer. This time-line is consistent with analysis of forward-looking behavior in health insurance choices; see Aron-Dine et al. (2012).
For example, in 2007 a drug bill of $14,175 was required for an enrollee with full gap coverage to reach the CCT. Consequently, this enrollee co-paid at a 25 percent rate rather than the Standard plan 5 percent rate for drug bills between $5451.25 and $14,175, eventually repaying all the benefits received from gap coverage.
Insurers can readily retrieve complete information on the FBD and premiums of rivals from public data, so that the legislative and administrative confidentiality restrictions of CMS do little to advance the stated objective of protecting “corporate privacy”. However, they are quite effective in impeding scientific research on the impact of formulary and benefit rules on the health of Medicare enrollees.
Einav et al. (2013) analyze the bunching of annual drug bills at the donut hole thresholds in more detail; they argue that it provides evidence of non-zero price responses in prescription drug demand.
In the context of Medicare Part D, “creditable coverage” is coverage that is expected to pay on average as much as the standard Medicare prescription drug coverage.
Table 3 puts the 15.1 percent rate in context by providing breakdowns for 2006, 2007, and 2008 by various enrollment pathways.
Our calculations are based on 253,080 beneficiaries enrolled in Gold and 23,468 enrolled in Platinum plans and 3,412,891 beneficiaries enrolled in Part D stand-alone PDP; see Table 4 (Panel A).
In practice, CMS reports that only about 20 percent of Part D enrollees consult Plan Finder, and it is unclear how much information the remainder obtain indirectly.
The degree of risk aversion estimated from model (3) with the rational decision rule puts a value of $97.17 on a $1000 bet that pays off with probability 0.1 and has expected value $100.
The averages are calculated for people in the middle 80% of the distribution of prior year total drug bills. The premium differences that the calculations indicate people should be willing to pay for gold coverage rise sharply with prior year total drug bill, then fall at very high prior year total drug bills when the higher catastrophic coverage threshold for gold plans offsets the advantages of gap coverage.
These expectations and averages could be taken over demographic subpopulations, classified say by gender or age bracket, to evaluate the quality of choices of the various groups, but we leave these extensions for future research.
In an earlier version of this paper (Heiss et al., 2012) we discuss additional cases that are not reported here.
As noted in section 4.1, we find no consistent evidence for risk aversion, and consequently we set α = 0 and omit the final term in the certainty equivalent expected CIC = ECIC(k,Xt-1) + αVAOOP(k,Xt-1) criterion that would come out of CARA expected utility maximization in the general case.
In their analysis of plan choice in the Massachusetts Connector, Ericson and Starc (2012) document that consumers “gravitate towards the cheapest and least generous plans” (p. 497). They argue that this behavior might reflect the use of a simple “choose the cheapest plan” heuristic but it could also reflect preference heterogeneity.
The 22 CCW conditions include chronic obstructive pulmonary disease and brochiectasis, depression, diabetes, glaucoma, heart failure, hip/pelvic fracture, hyperlipidemia, hypertension, ischemic heart disease, osteoporosis, rheumatoid arthritis/osteoarthritis, stroke/transient ischemic attack, breast cancer, colorectal cancer, prostate cancer, lung cancer, and endometrial cancer. For detailed information on the construction of the chronic conditions, see www.ccwdata.org/cs/groups/public/documents/document/ccw_conditioncategories2011.pdf. We code the presence of each chronic condition as of December 31 of the year that plan choices are made.
For more information on the construction of the HCC score, see http://www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/Risk_adjustment.html consumers could have adopted, the measures that we have argued are most appropriate for assessing the quality of Part D plan choices.
A consumer using Plan Finder should have positive average excess spending relative to the decision rule of a fully rational consumer, but this is not necessarily true for our implementation of a “rational” benchmark. Further, shares with positive excess spending are not necessarily monotone in levels of expected excess spending.
Iyengar and Kemenica (2010) report similar effects of the size of the choice set in a study of 401(k) investment plans.
In real-world choice situations, the negative effect of age on choice quality might be partially compensated by the fact that individuals are more likely to ask others for help as they get older, in particular as their cognitive ability declines.
Some information on socioeconomic and health disparities in Part D enrollment can be obtained from Levy and Weir (2010) and Winter et al. (2006). One feature of the Part D enrollment and plan choice process is that as the result of family, Medicare, and Medicaid interventions, disadvantaged individuals often have help in making Part D decisions, so that access to informed help may be more important than individual SES and cognitive ability.
For CCW documentation, see http://www.ccwdata.org/index.htm.; for details on conditions, exceptions, and references see www.ccwdata.org/cs/groups/public/documents/document/ccw_conditioncategories2011.pdf.
CWW in 2010 added hypertension, acquired hypothyroidism, anemia, asthma, benign prostatic hyperplasia, and hyperlipidemia to their health condition list. In this study, we have incorporated hypertension, but not the remaining additions. We follow CCW in treating as separate conditions Alzheimer’s Disease, and the broader category of Alzheimer’s Disease, related conditions, and senile dementia.
RxNorm is documented at https://www.nlm.nih.gov/research/umls/rxnorm/.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Florian Heiss, University of Düsseldorf.
Adam Leive, University of Pennsylvania.
Daniel McFadden, University of California, Berkeley and University of Southern California.
Joachim Winter, University of Munich.
References
- Abaluck JT, Gruber J. Choice inconsistencies among the elderly: Evidence from plan choice in the Medicare Part D program. American Economic Review. 2011;101:1180–1210. doi: 10.1257/aer.101.4.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Driscoll JC, Gabaix X, Laibson D. The age of reason: Financial decisions over the life cycle and implications of regulation. Brookings Papers on Economic Activity. 2009 Fall;:51–101. [Google Scholar]
- Aron-Dine A, Einav L, Finkelstein A, Cullen MR. Working Paper No. 17802. National Bureau of Economic Research (NBER); 2012. Moral Hazard in Health Insurance: How Important Is Forward Looking Behavior? [Google Scholar]
- Bach PB, McClellan MB. A prescription of a modern Medicare program. New England Journal of Medicine. 2005;353:2733–2735. doi: 10.1056/NEJMp058249. [DOI] [PubMed] [Google Scholar]
- Bach PB, McClellan MB. The first months of the prescription-drug benefit: A CMS update. New England Journal of Medicine. 2006;354:2312–2314. doi: 10.1056/NEJMp068108. [DOI] [PubMed] [Google Scholar]
- Besedeš T, Deck C, Sarangi S, Shor M. Age effects and heuristics in decision making. Review of Economics and Statistics. 2012;94:580–595. doi: 10.1162/REST_a_00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besedeš T, Deck C, Sarangi S, Shor M. Decision-making strategies and performance among seniors. Journal of Economic Behavior and Organization. 2012;81:524–533. doi: 10.1016/j.jebo.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buntin MB, Damberg C, Haviland A, Kapur K, Lurie N, McDevitt R, Marquis MS. Consumer-directed health care: Early evidence about effects on cost and quality. Health Affairs. 2006;25:w516–w530. doi: 10.1377/hlthaff.25.w516. [DOI] [PubMed] [Google Scholar]
- Centers for Medicare and Medicaid Services. Technical report. Centers for Medicare and Medicaid Services (CMS); Washington, DC: 2005. Instructions for the Part D payment demonstration. [Google Scholar]
- Centers for Medicare and Medicaid Services. [Accessed January 21, 2012];Statistical Supplement. 2012 Available at: https://www.cms.gov/MedicareMedicaidStatSupp/
- Congressional Budget Office. Technical Report. Congressional Budget Office (CBO); Washington, DC: 2004. A detailed description of CBO’s cost estimates for the Medicare prescription drug benefit. [Google Scholar]
- Cutler DM, Reber SJ. Paying for health insurance: The trade-off between competition and adverse selection. Quarterly Journal of Economics. 1998;113:433–466. [Google Scholar]
- Cutler DM, Zeckhauser RJ. The anatomy of health insurance. In: Culyer AJ, Newhouse JP, editors. Handbook of Health Economics. Vol. 1. Amsterdam: Elsevier; 2000. pp. 563–643. [Google Scholar]
- Domino ME, Stearns SC, Norton EC, Yeh WS. Why using current medications to select a Medicare Part D plan may lead to higher out-of-pocket payments. Medical Care Research and Review. 2008;65(1):114–126. doi: 10.1177/1077558707307577. [DOI] [PubMed] [Google Scholar]
- Duggan M, Healy P, Scott Morton F. Providing prescription drug coverage to the elderly: America’s experiment with Medicare Part D. Journal of Economic Perspectives. 2008;22(4):69–92. doi: 10.1257/jep.22.4.69. [DOI] [PubMed] [Google Scholar]
- Einav L, Finkelstein A, Schrimpf P. Unpublished manuscript. Stanford University, University of British Columbia, and Massachusetts Institute of Technology; 2013. The response of drug expenditure to contract design in Medicare Part D. [Google Scholar]
- Engelhardt GV, Gruber J. Medicare Part D and the financial protection of the elderly. American Economic Journal: Economic Policy. 2011;3(4):77–102. [Google Scholar]
- Ericson KM. Consumer Inertia and Firm Pricing in the Medicare Part D Prescription Drug Insurance Exchange. American Economic Journal: Economic Policy. 2012 forthcoming. [Google Scholar]
- Ericson KM, Starc A. Heuristics and heterogeneity in health insurance exchanges: Evidence from the Massachusetts Connector. American Economic Review: Papers and Procedeedings. 2012;102(3):493–497. doi: 10.1257/aer.102.3.493. [DOI] [PubMed] [Google Scholar]
- Frank RG, Newhouse JP. Discussion Paper No. 2007-03, The Hamilton Project. Brookings Institution; Washington, DC: 2007. Mending the Medicare prescription drug benefit: Improving consumer choices and restructuring purchasing. [Google Scholar]
- Goldman DP, Joyce GF. Medicare Part D: A successful start with room for improvement. Journal of the American Medical Association. 2008;299(16):1954–1955. doi: 10.1001/jama.299.16.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman DP, Joyce GF, Escarce JJ, Pace JE, Solomon MD, Laouri M, Landsman PB, Teutsch SM. Pharmacy benefits and the use of drugs by the chronically ill. Journal of the American Medical Association. 2004;291(19):2344–2350. doi: 10.1001/jama.291.19.2344. [DOI] [PubMed] [Google Scholar]
- Goldman DP, Joyce GF, Vogt WB. Part D formulary and benefit design as a risk steering mechanism. American Economic Review, Papers and Proceedings. 2011;101(3):382–386. doi: 10.1257/aer.101.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman J. Policy Brief 2006-PB-20. Networks Financial Institute, Indiana State University; 2006. Consumer directed health care. [Google Scholar]
- Gruber J. Covering the uninsured in the United States. Journal of Economic Literature. 2008;46:571–606. [Google Scholar]
- Handel B. Unpublished manuscript. University of California; Berkeley: 2012. Adverse selection and inertia in health insurance markets: When nudging hurts. [DOI] [PubMed] [Google Scholar]
- Handel B, Kolstad JT. Unpublished manuscript. University of California, Berkeley, and The Wharton School, University of Pennsylvania; 2013. Health insurance for humans: Information frictions, plan choice, and consumer welfare. [Google Scholar]
- Heiss F, McFadden D, Winter J. Who failed to enroll in Medicare Part D, and why? Early results. Health Affairs. 2006;25:w344–w354. doi: 10.1377/hlthaff.25.w344. [DOI] [PubMed] [Google Scholar]
- Heiss F, McFadden D, Winter J. Working Paper No. 15329. National Bureau of Economic Research (NBER); 2009. Regulation of health insurance markets: Lessons from enrollment, plan choice, and adverse selection in Medicare Part D. [Google Scholar]
- Heiss F, McFadden D, Winter J. Mind the gap! Consumer perceptions and choices of Medicare Part D prescription drug plans. In: Wise DA, editor. Research Findings in the Economics of Aging. Chicago and London: Chicago University Press; 2010. pp. 413–481. [Google Scholar]
- Heiss F, McFadden D, Winter J. The demand for Medicare Part D prescription drug coverage: Evidence from four waves of the Retirement Perspectives Survey. In: Wise DA, editor. Explorations in the Economics of Aging. Chicago and London: Chicago University Press; 2011. pp. 159–182. [Google Scholar]
- Heiss F, Leive A, McFadden D, Winter J. Working Paper No. 18166. National Bureau of Economic Research (NBER); 2012. Plan Selection in Medicare Part D: Evidence from Administrative Data. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar SS, Kamenica E. Choice proliferation, simplicity seeking, and asset allocation. Journal of Public Economics. 2010;94:530–539. [Google Scholar]
- Johnson EJ, Hassin R, Baker T, Bajger AT, Treuer G. Unpublished manuscript. Columbia University; 2013. Can consumers make affordable care affordable? The value of choice architecture. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketcham JD, Lucarelli C, Miravete EJ, Roebuck MC. Sinking, swimming, or learning to swim in Medicare Part D. American Economic Review. 2012;102:2639–2673. doi: 10.1257/aer.102.6.2639. [DOI] [PubMed] [Google Scholar]
- Kling JR, Mullainathan S, Shafir E, Vermeulen L, Wrobel MV. Comparison friction: Experimental evidence from Medicare drug plans. Quarterly Journal of Economics. 2012;127(1):199–235. doi: 10.1093/qje/qjr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy H, Weir D. Take-up of Medicare Part D: Results from the Health and Retirement Study. Journal of Gerontology: Social Sciences. 2010;65B:492–501. doi: 10.1093/geronb/gbp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucarelli C, Prince J, Simon K. The welfare impact of reducing choice in Medicare part D: A comparison of two regulation strategies. International Economic Review. 2012;53:1155–1177. [Google Scholar]
- McGuire T, Newhouse J, Sinaiko A. An economic history of Medicare Part C. The Milbank Quarterly. 2011;89(2):289–332. doi: 10.1111/j.1468-0009.2011.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse JP. Consumer-directed health plans and the RAND health insurance experiment. Health Affairs. 2004;23(6):107–113. doi: 10.1377/hlthaff.23.6.107. [DOI] [PubMed] [Google Scholar]
- Perry M, Dulio A, Cubanski J. Kaiser Family Foundation Report. 2006. Voices of beneficiaries: Medicare Part D insights and observations one year later. [Google Scholar]
- Sinaiko AD, Hirth RA. Consumers, health insurance, and dominated choices. Journal of Health Economics. 2011;30:450–457. doi: 10.1016/j.jhealeco.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Sinaiko AD, Ross-Degnan D, Soumerai SB, Lieu T, Galbraith A. The experience of Massachusetts shows that consumers will need help in navigating insurance exchanges. Health Affairs. 2013;32(1):78–86. doi: 10.1377/hlthaff.2012.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J, Balza R, Caro F, Heiss F, Jun B, Matzkin R, McFadden D. Medicare prescription drug coverage: Consumer information and preferences. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(20):7929–7934. doi: 10.1073/pnas.0601837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Zhang Y. The vast majority of Medicare Part D beneficiaries still don’t choose the cheapest plans that meet their medication needs. Health Affairs. 2012;31(10):2259–2265. doi: 10.1377/hlthaff.2012.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]