Figure 2.
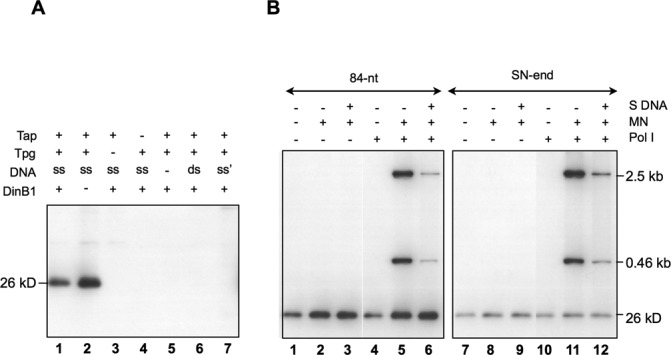
In vitro deoxynucleotidylation and oligonucleotide elongation. (A) Deoxynucleotidylation in the presence or absence of DinB1. The reaction mixture contained 0.165 μM [α-32P]-dCTP, 1.5% glycerol, 75 mM NaCl, 75 mM imidazole, and various concentrations of proteins in K buffer (10 mM Tris–HCl pH 7.5, 7 mM Mg2+, 0.1 mM DTT). The final concentrations of proteins used were 135 nM of Tap, 280 nM of Tpg and 500 nM of DinB1. The template was a 200-bp PCR-generated S. lividans telomere DNA digested by HaeIII digestion, which left the very end of the telomere sequence exposed (‘ds’), or its heat denatured form (‘ss’) in a final concentration of 9 nM. The products were separated by 12% SDS-PAGE. The radioactively labeled deoxynucleotidylated Tpg exhibited a mobility of a 26 kD protein. In lane 7, the fragment (‘ss’) was the 200-bp PCR product without HaeIII digestion. (B) Deoxynucleotidylation and oligonucleotide extension in the absence of DinB1. The reaction mixture contained 0.165 μM [α-32P]-dCTP, 1% glycerol, 50 mM NaCl, 50 mM imidazole, 60 nM Tap, and 600 nM Tpg (and no DinB1). In some cases, the following materials were added: two MulI–NdeI restriction fragments (‘MN’; 2.5- and 0.46-kb), salmon sperm DNA (‘S DNA’, 2 μg) and E. coli Pol I (10−2 units). The template was an 84-nt telomere DNA (50 nM) or a 460-bp SalI–NdeI (‘SN-end’) fragment containing the telomere DNA of S. lividans with the end exposed at the SalI cleavage site at a concentration of 1.35 nM.
