Figure 2.
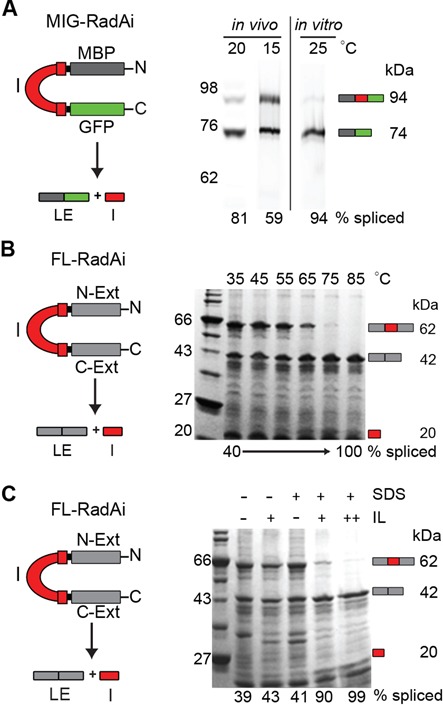
RadA intein splicing is regulated by its native exteins in temperature and solution-dependent manner. (A) RadA intein splicing in foreign exteins is efficient even at low temperatures. The RadA intein was cloned into the non-native extein context (pMIG-RadAi; Table 2) and the recombinant protein was overexpressed in BL21 Star (DE3). The MIG-RadAi precursor (left) consists of the RadA intein (I; red) with short native exteins fused to MBP (dark gray) and GFP (green). The precursor (94 kDa) and the ligated exteins (LE; 74 kDa) were visualized by in-gel GFP fluorescence. More than 50% of precursor was spliced in vivo at 15°C and >80% was spliced at 20°C. The MIG-RadAi precursor recovered from a 15°C induction spliced efficiently (94% splicing) in vitro at 25°C within 1 h. (B) RadA intein splicing in the native exteins is inefficient at low temperatures, but efficient at high temperature. In the FL-RadAi precursor (left) the RadA intein (red) is flanked by its native exteins (N-Ext and C-Ext; gray). RadA intein splicing of the Ni-NTA purified FL-RadAi precursor was visualized in a Coomassie blue-stained gel. Accumulation of the spliced intein (I; 20 kDa) and the ligated exteins (LE; 42 kDa) and disappearance of the FL-RadAi precursor (62 kDa) were observed at high temperatures. A plot of the data from Figure 2B is shown in Figure 3B (middle panel). (C) Solution effects on RadA intein splicing in the native exteins. RadA intein splicing of FL-RadAi is shown in the presence 1.25% (+) and 2.5% (++) of ionic liquid (IL) 1-butyl-3-methylimidazolium chloride and 0.5% (+) SDS for samples incubated at 25°C for 30 min.
