Figure 6.
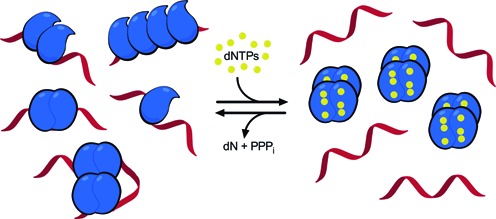
Possible binding modes of SAMHD1 to ssRNA and a model for the regulation of SAMHD1 dNTPase and RNA binding activities. In the low dNTP environment of resting immune cells, SAMHD1 exists in monomeric and dimeric forms that have high affinity for single-stranded RNA but possess no dNTPase activity. The figure depicts possible structures for RNA complexes with one, two and four monomers of SAMHD1 that are consistent with the biochemical, mutagenesis and AFM findings. The binding footprint for two adjacent monomers is ∼60 nt (Supplementary Figure S14), and two hypothetical binding modes for four monomers are depicted, consistent with chemical crosslinking (Figure 5A) and the elongated or globular complexes observed in AFM images (Figure 5E). Complexes of these types could form on HIV or LINE-1 ssRNA genomes. When dNTP levels increase, SAMHD1 can shift to its tetrameric form with high dNTPase activity but little affinity for single-stranded nucleic acids. Upon hydrolysis of available dNTP substrates by the activated SAMHD1 tetramers, the system will revert to the monomeric and dimeric states that are competent for ssRNA binding.
