Highlight
To antagonize the developmental process initiated by Rhodococcus fascians and in response to the bacterial cytokinins, Arabidopsis activates its strigolactone response, partially suppressing shoot branching in the rosette.
Key words: Apical dominance, Gram-positive phytopathogen, witches’ broom.
Abstract
Leafy gall syndrome is the consequence of modified plant development in response to a mixture of cytokinins secreted by the biotrophic actinomycete Rhodococcus fascians. The similarity of the induced symptoms with the phenotype of plant mutants defective in strigolactone biosynthesis and signalling prompted an evaluation of the involvement of strigolactones in this pathology. All tested strigolactone-related Arabidopsis thaliana mutants were hypersensitive to R. fascians. Moreover, treatment with the synthetic strigolactone mixture GR24 and with the carotenoid cleavage dioxygenase inhibitor D2 illustrated that strigolactones acted as antagonistic compounds that restricted the morphogenic activity of R. fascians. Transcript profiling of the MORE AXILLARY GROWTH1 (MAX1), MAX2, MAX3, MAX4, and BRANCHED1 (BRC1) genes in the wild-type Columbia-0 accession and in different mutant backgrounds revealed that upregulation of strigolactone biosynthesis genes was triggered indirectly by the bacterial cytokinins via host-derived auxin and led to the activation of BRC1 expression, inhibiting the outgrowth of the newly developing shoots, a typical hallmark of leafy gall syndrome. Taken together, these data support the emerging insight that balances are critical for optimal leafy gall development: the long-lasting biotrophic interaction is possible only because the host activates a set of countermeasures—including the strigolactone response—in reaction to bacterial cytokinins to constrain the activity of R. fascians.
Introduction
Leafy gall syndrome is an infectious plant disease that affects a wide range of plants, primarily dicotyledonous herbs (for recent reviews, see Stes et al., 2011b, 2013). The pathology is caused by the Gram-positive actinomycete Rhodococcus fascians and is characterized by the induction of multiple shoots, of which further outgrowth is inhibited. These shoots arise from the activation of dormant axillary meristems combined with the de novo formation of additional meristems (de O Manes et al., 2001; Simón-Mateo et al., 2006). The main virulence factor of R. fascians strain D188 is a cytokinin mix for which the biosynthetic machinery is encoded by the fas operon located on a linear virulence plasmid, pFiD188 (Pertry et al., 2009, 2010; Francis et al., 2012). The central gene of the operon, fasD, encodes an isopentenyltransferase that mediates the first dedicated step in cytokinin production (Pertry et al., 2009, 2010). Although the plasmid-free derivative of strain D188, strain D188-5, also secretes low levels of cytokinins, they are insufficient to cause the disease (Pertry et al., 2009, 2010). The perception of the bacterial cytokinins by plants is absolutely essential for leafy gall induction on all hosts because it stimulates cell proliferation, prevents tissue maturation, and converts infected regions into sink tissues (Depuydt et al., 2008, 2009a , 2009b ). Moreover, in Arabidopsis, the fas cytokinins also trigger increased production of other plant growth regulators, such as polyamines and auxins. More specifically, indole-3-acetic acid and putrescine have been shown to play accessory roles during symptom formation by activating meristem initiation and targeting expression of D3-type cyclins, respectively (Stes et al., 2011a, 2012). In this manner, the relatively low amounts of cytokinins secreted by R. fascians can profoundly alter plant development. Nevertheless, additional hormones, such as abscisic acid and gibberellins, may be implicated in symptom development as well (Simón-Mateo et al., 2006; Depuydt et al., 2008), but their exact role in the pathology remains to be assessed. In Arabidopsis, R. fascians infection leads to delayed senescence, loss of apical dominance, activation of dormant axillary meristems, and formation of stunted inflorescences from dwarfed rosettes, altogether resulting in a bushy appearance (de O Manes et al., 2004; Stes et al., 2011b ). Interestingly, these aspects of leafy gall syndrome resemble the phenotype of mutants impaired in strigolactone biosynthesis and/or sensing.
Strigolactones are apocarotenoids that have been classified as plant hormones. Together with auxins and cytokinins, they take a central position in the control of shoot branching (Gomez-Roldan et al., 2008; Umehara et al., 2008). Strigolactones have also been associated with other aspects of plant development (for recent reviews see Xie et al., 2010; Brewer et al., 2013; Waldie et al., 2014). For instance, they control light-dependent photomorphogenesis during seed germination, influence root architecture, impact senescence, and affect flower development (Snowden et al., 2005; Shen et al., 2007; Agusti et al., 2011; Domagalska and Leyser, 2011; Kapulnik et al., 2011; Ruyter-Spira et al., 2011; Rasmussen et al., 2012; Shen et al., 2012). Moreover, strigolactones appear to be linked to diverse abiotic stresses, such as nutrition (Bonneau et al., 2013; Marzec et al., 2013), drought, high salinity (Bu et al., 2014; Ha et al., 2014), and light stress (González-Pérez et al., 2011; Jia et al., 2014). However, the oldest known function of strigolactones is as rhizospheric host detection cues for root-parasitic plants and symbiotic arbuscular mycorrhizal fungi (Cook et al., 1966; Akiyama et al., 2005; Matusova et al., 2005). More recently, strigolactones have also been implicated in other biotic interactions. For instance, strigolactones have been reported to affect nodule formation in diverse legumes upon interaction with their rhizobial partner (Soto et al., 2010; Liu et al., 2013; Foo et al., 2014; De Cuyper et al., 2015). In Oryza sativa (rice), the excess tillering observed after infection with rice grassy stunt virus has been associated with suppression of strigolactone biosynthesis and signalling genes (Satoh et al., 2013). Finally, strigolactones might play a direct or indirect role in plant defence in different fungal pathosystems (Dor et al., 2011; Torres-Vera et al., 2013).
Strigolactones are derived from carotenoids and the first biosynthesis steps occur in the plastids. The β-carotene isomerase DWARF27 (D27) mediates the conversion of all-trans-β-carotene to 9-cis-β-carotene (Alder et al., 2012), after which two carotenoid cleavage dioxygenases (CCDs), MORE AXILLARY GROWTH3 (MAX3) and MAX4 in Arabidopsis, cleave these intermediates to form carlactone. Carlactone moves from the plastids to the cytosol and undergoes oxidation by the cytosolic cytochrome P450 MAX1, resulting in the bioactive compounds (Booker et al., 2005; Waters et al., 2012; Abe et al., 2014). Strigolactones are thought to be produced mainly in the roots and to be transported upwards in the xylem to inhibit bud outgrowth (Beveridge, 2006; Kohlen et al., 2011). Two interacting proteins were shown to be central in strigolactone perception and signalling: D14/DAD2, an α/β-fold hydrolase proposed to be a strigolactone receptor (Waters et al., 2012; Hamiaux et al., 2012; Chevalier et al., 2014), and MAX2/D3/RMS4 that is part of a Skp-Cullin-F-box (SCF) E3 ligase (Stirnberg et al., 2002; Ishikawa et al., 2005; Johnson et al., 2006). The current understanding is that strigolactones are hydrolysed by D14/DAD2, upon which binding to SCFMAX2 is enhanced to trigger ubiquitination and target degradation of downstream signalling components, such as D53 and SLENDER1 in rice, and BRI1-EMS-SUPPRESSOR1 in Arabidopsis (Hamiaux et al., 2012; Jiang et al., 2013; Nakamura et al., 2013; Wang et al., 2013; Zhou et al., 2013). Downstream of this early signalling complex, the TEOSINTE BRANCHED1/CYCLOIDEA/PCF1 transcription factor BRANCHED1 (BRC1) might function as an integrator of different hormonal signals to control branching (Aguilar-Martínez et al., 2007; Braun et al., 2012; Dun et al., 2012).
Here, the importance of strigolactones in pathogen-induced changes in plant development is evaluated in leafy gall syndrome, a pathology with a strong link to apical dominance and bud outgrowth (de O Manes et al., 2004; Simón-Mateo et al., 2006). First, the phenotype induced by R. fascians strain D188 on wild-type Arabidopsis plants was compared with that provoked on the four max and the brc1 mutants. Then, in a pharmacological approach, the importance of the endogenous strigolactone levels on symptom development was assessed by adding the synthetic racemic strigolactone mixture GR24 (Besserer et al., 2006; Scaffidi et al., 2014) or the CCD inhibitor D2 (Sergeant et al., 2009). The expression profiles were determined of the four MAX genes and of BRC1 in infected tissues of wild-type Columbia-0 (Col-0) plants and different mutants previously shown to be impaired in symptom development. Finally, diverse approaches were taken to assess the strigolactone levels in infected tissues and the impact of the bacterial cytokinins on the observed transcriptional modulations was analysed. Based on the obtained results, the latest model on the molecular basis of leafy gall formation (Stes et al., 2012) was extended.
Materials and methods
Plant material, sampling, and infection conditions
Arabidopsis thaliana (L.) Heynh., accession Col-0 was used throughout the experiments. Seeds of the max mutant and the max β-glucuronidase (GUS) lines were kindly provided by Ottoline Leyser (University of Cambridge, UK), the brc1 mutant by Pilar Cubas (Universidad Autónoma de Madrid, Spain), the tryptophan aminotransferase1-1 (taa1-1) taa1-related2-1 (tar2-1) [weak ethylene insensitive8-1 (wei8-1) wei2-1] mutant by Hélène Boisivon (VIB-Ghent University, Belgium), and the Arabidopsis histidine kinase3 (ahk3) ahk4 mutant by Tatsuo Kakimoto (Osaka University, Japan).
The seeds were sterilized and sown on half-strength Murashige and Skoog medium in a growth chamber under a 16-h/8-h light/dark photoperiod at 21±2°C. The R. fascians strains used were the pathogenic strain D188, containing the linear virulence plasmid pFiD188, and its plasmid-free non-pathogenic derivative D188-5 (Desomer et al., 1988). These strains were grown in liquid yeast extract broth at 28°C under gentle agitation for 2 days, then diluted 100-fold in fresh medium, and allowed to grow overnight. Prior to infection, the cultures were washed and concentrated 4-fold by resuspending the bacterial pellets in sterile distilled H2O. Arabidopsis plants were infected 14 days after germination by local application of a drop of bacterial culture to the shoot apical meristem. At different time points post infection [0, 4, 7, 14, and 24 days post infection (dpi)], shoot samples for quantitative reverse-transcription-polymerase chain reaction (qRT-PCR) analysis were collected after removal of roots and flower stalks and were snap-frozen in liquid nitrogen.
Chemical treatments
GR24 (obtained from Binne Zwanenburg, Radboud University Nijmegen, The Netherlands) was dissolved in acetone and D2 (ChemBridge Corporation; www.chembridge.com/) in dimethylsulfoxide (DMSO). The cytokinins (OlChemIm Ltd.; www.olchemin.cz) were dissolved in DMSO and supplemented to half-strength Murashige and Skoog medium at concentrations of 1 µM each for the mix of 2-isopentenyladenine (2-iP), trans-zeatin (tZ), cis-zeatin (cZ), and their 2-methylthio (2MeS) derivatives, or 10 μM for tZ. To this medium, 14-day-old plants were transferred and sampled as described above for qRT-PCR analyses after 7 days of treatment.
RNA isolation, cDNA synthesis, and gene expression analysis
For per sample, 100mg of shoot tissue was collected and ground in liquid nitrogen. For each experiment, three biological repeats were sampled. Extraction and reverse transcription of RNA were performed as described by Stes et al. (2011a ). All qRT-PCR reactions were done under the same standardized conditions: initial denaturation at 95°C for 5min, followed by 45 cycles at 95°C for 10 s, 60°C for 10 s, and 72°C for 10 s. Analysis of the data, normalized against ACTIN2, was as previously reported (Stes et al., 2011a ). The primer sequences are given in Table 1.
Table 1.
Primers used for qRT-PCR amplifications
| Gene | AGI | Sense | Primer sequence | Reference |
|---|---|---|---|---|
| ACT2 | At3g18780 | Forward | GGCTCCTCTTAACCCAAAGGC | Simón-Mateo et al. (2006) |
| Reverse | CACACCATCACCAGAATCCAGC | |||
| MAX1 | At2g26170 | Forward | AGACTGAGTGGACAACTTAATGAG | This work |
| Reverse | GCAGAGCCAGCAAGAAGATG | |||
| MAX2 | At2g42620 | Forward | CTCACCTCACTATCCGTGGCAAC | This work |
| Reverse | CGATTGGGAGAGAAGCGAGAAGAG | |||
| MAX3 | At2g44990 | Forward | CCTCGTCCGTACTTGGTCTAC | This work |
| Reverse | TCGTCCTCTTCTTCTCCTTCTTC | |||
| MAX4 | At4g32810 | Forward | AGAAGGTGGAAGGTGAGAG | This work |
| Reverse | TGACGAGTGTGGAGTAGC | |||
| BRC1 | At3g18550 | Forward | CTTCAGCAGCGGCGATGAG | This work |
| Reverse | TTCCTCTTGTTTCGGTCGTGTTAG |
Preparation of ethyl acetate extracts and liquid chromatography-tandem mass spectrometry analysis
Entire mock-inoculated and D188-infected Col-0 and max4 plants were harvested at 14 dpi and 48 dpi, pooled per treatment in Erlenmeyer flasks (between 3.05g and 7.53g), submerged in ethyl acetate, and rotated at 4°C for 2 days. After filtration, the ethyl acetate extracts were washed with 0.2M KH2PO4 to remove acidic compounds, dried over anhydrous Na2SO4, and filtered. The extracts were dried under a nitrogen flow at room temperature. The liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis was done as described (Yoneyama et al., 2007).
Orobanche minor seed germination assay
Orobranche minor seeds were kindly provided by Gerda Cnops (Institute for Agricultural and Fisheries Research, Merelbeke, Belgium). The seeds were surface sterilized with 70% (v/v) EtOH containing 0.05% (v/v) sodium dodecyl sulfate for 5min and then washed with 95% (v/v) EtOH for 5min and air-dried. For the preconditioning, the seeds were sprinkled on a filter paper humidified with 1ml of sterile H2O in a Petri dish (5cm), sealed with parafilm, and kept in the dark at 24°C for 7 days. Excess water was removed as much as possible. For the positive control, 1ml of 0.1 µM or 1 µM GR24 solution was added; for the negative control, 1ml of H2O was added. Samples of the ethyl acetate extracts corresponding to 18.5mg of plant tissue were dried, dissolved in 10 µl acetone, and diluted to 1ml with water. The sample tubes were left open in the laminar flow for 30min to allow evaporation of the acetone, whereafter the samples were added to the seeds. The Petri dishes were resealed with parafilm and incubated in the dark at 24°C. After 7 days, the germination percentage was determined. All incubations were done in triplicate.
Statistical analysis
Because assumptions for parametric tests were not met, differences in axillary activation were analysed with the Kruskal–Wallis test. When significant (P < 0.05), the Mann–Whitney U test for pairwise analysis, corrected with a sequential Bonferroni correction for multiple pairwise comparisons, was used.
All qRT-PCR reactions were run in triplicate, and each experiment was repeated three times. Data were compared by paired, two-tailed Student’s t-tests (criterion significance P < 0.05 for all comparisons).
Results
Strigolactone-related mutants display enhanced symptoms upon R. fascians infection
Because of the partial resemblance between the phenotype of Arabidopsis plants infected with R. fascians and that of max mutants, the responsiveness of the max1-1, max2-1, max3-9, max4-1, and brc1-2 mutants (Sorefan et al., 2003; Booker et al., 2004; Aguilar-Martínez et al., 2007; Stirnberg et al., 2007) towards R. fascians infection was evaluated and the phenotype of the rosette was compared to that of infected wild-type plants.
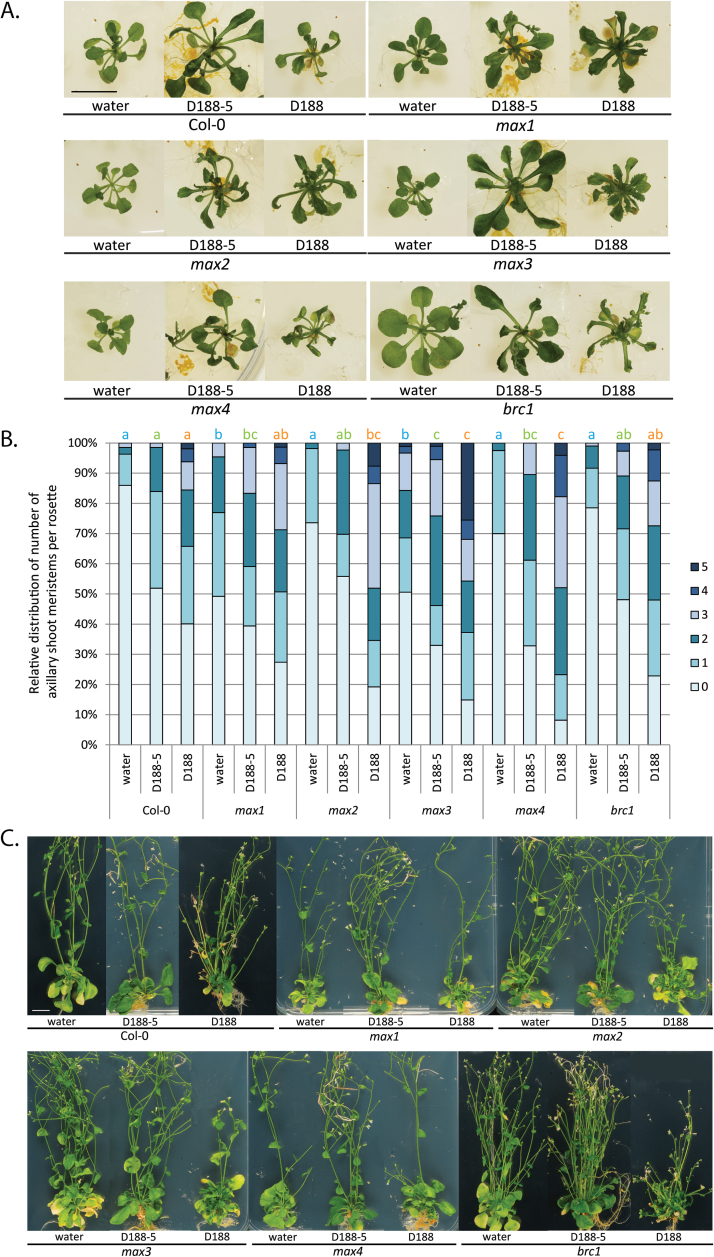
Upon infection of 2-week-old wild-type Col-0 plants with the virulent strain D188, newly developed leaves show thickened veins and serrated margins and eventually multiple axillary shoots arise from the heart of the rosette. This shoot proliferation ultimately results in bushy and often stunted plants (de O Manes et al., 2004; Depuydt et al., 2008; Stes et al., 2011a; 2012) (Fig. 1A, C). Strain D188-5, a non-pathogenic plasmid-free derivative of strain D188, does not provoke leafy gall syndrome (Stes et al., 2011a ), but has a transient phytostimulatory effect on Arabidopsis (our unpublished data) (Fig. 1A, C). Strigolactone biosynthesis as well as the signalling mutants were all responsive towards strain D188, but, interestingly, the axillary activation in the mutants appeared to be more pronounced than that in the infected Col-0 plants (Fig. 1A), resulting in more severe bushiness of the rosettes of the mutant plants at the end of the experiment (Fig. 1C). Just as in wild-type plants, infection of the strigolactone mutants with strain D188-5 initially stimulated growth to some extent (Fig. 1A), but at the end of the experiment, no developmental changes occurred that differed from the mock-infected controls (Fig. 1C).
Fig. 1.
Symptom development in Arabidopsis Col-0 and strigolactone-related mutants upon infection with R. fascians. (A) Phenotype of representative plants mock-inoculated with water and infected with the non-pathogenic strain D188-5 or the virulent strain D188 at 10 dpi showing the outgrowth of shoots in axils of rosette leaves. All images were taken at the same magnification. Bar = 1cm. (B) Quantification of axillary activation (number of outgrowing shoot meristems per plant ranging between 0 and 5) at 10 dpi on at least 60 individual plants per treatment. Bars with different letters indicate a significant difference between treatments after a Kruskal–Wallis and Mann–Whitney U analysis followed by a Bonferroni correction for multiple comparisons (P = 0.05/n, with n = number of comparisons) (blue, water; green, D188-5; and orange, D188). (C) Phenotype of representative plants mock-inoculated with water and infected with the non-pathogenic strain D188-5 or the virulent strain D188 at 42 dpi. The effect of R. fascians occurs mainly in the rosette, evidenced by a bushy appearance. All images were taken at the same magnification. Bar = 1cm.
At 10 dpi (Fig. 1A), the axillary activation degree was scored under the binocular by counting the number of outgrowing shoot meristems in the axillary regions of the rosette leaves. For the mock-inoculated controls, only max1 and max3 exhibited significantly stronger loss of apical dominance than the wild-type plants at this time point (Fig. 1B) (Stirnberg et al., 2002). In all plants tested, D188-5 infection induced axillary activation, possibly owing to its transient phytostimulatory effect (Fig. 1A, B). Whereas the response in the strigolactone signalling mutants was significantly higher than in wild-type plants, the axillary activation triggered by D188-5 was even higher in the three biosynthesis mutants (Fig. 1A, B). Nevertheless, of all treatments, strain D188 provoked the strongest axillary activation in all plants tested (Fig. 1A, B). The reaction of max1 and brc1 was comparable although more pronounced than in wild-type plants (Fig. 1A, B). The most significant differences were counted for max2 and especially max3 and max4 (Fig. 1A, B), possibly suggesting that MAX2-independent pathways might contribute to the observed phenotype. Altogether, the increased developmental response upon infection of all tested strigolactone mutants compared to wild-type plants (Fig. 1A, B) illustrates their hypersensitivity towards the R. fascians signals.
The efficiency of symptom development is determined by the strigolactone level
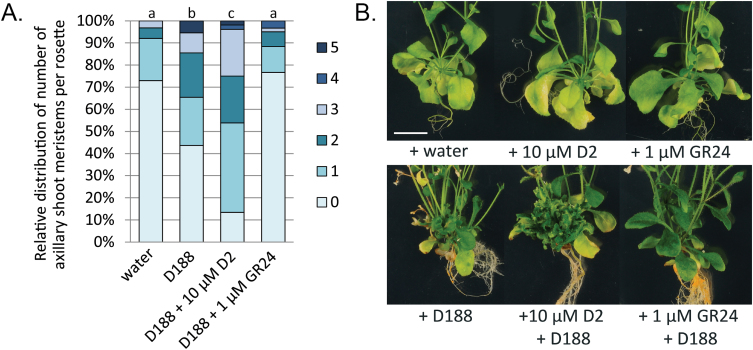
The data described above indicate that interference with stri-golactone biosynthesis and signalling has a positive impact on R. fascians-induced symptom development. To confirm this finding, 2-week-old wild-type Col-0 plants were transferred to media supplemented either with D2 (10 µM), an inhibitor of MAX3 and MAX4 activity, or with GR24 (1 µM), a synthetic racemic strigolactone mixture. These plants were then immediately infected with R. fascians strain D188. At 10 dpi, D2-inhibited strigolactone biosynthesis significantly stimulated the axillary activation triggered by strain D188 (Fig. 2A) and positively affected leafy gall formation, as evidenced by the extreme bushiness of the rosette at the end of the experiment (Fig. 2B). By contrast, GR24 reduced the axillary activation triggered by strain D188 to such an extent that the significant difference with the mock-infected control could no longer be observed (Fig. 2A). Consequently, in the presence of GR24, leafy gall formation was almost completely prevented (Fig. 2B). These results imply that the efficiency of symptom development is determined by the endogenous strigolactone levels.
Fig. 2.
Symptom development in Arabidopsis Col-0 after infection with R. fascians strain D188 and simultaneous treatment with 10 µM D2 or1 µM GR24 showing that the strigolactone level determines the efficiency of symptom development. (A) Quantification of axillary activation (number of outgrowing shoot meristems per plant ranging between 0 and 5) at 10 dpi on at least 30 plants per treatment. Letters indicate statistical differences per treatment after a Kruskal–Wallis and Mann–Whitney U analysis followed by a Bonferroni correction for multiple comparisons (P = 0.05/n, with n = number of comparisons). (B) Phenotype of representative plant rosettes at 42 dpi. All images were taken at the same magnification. Bar = 1cm.
Infection with R. fascians transcriptionally activates the strigolactone response in Arabidopsis
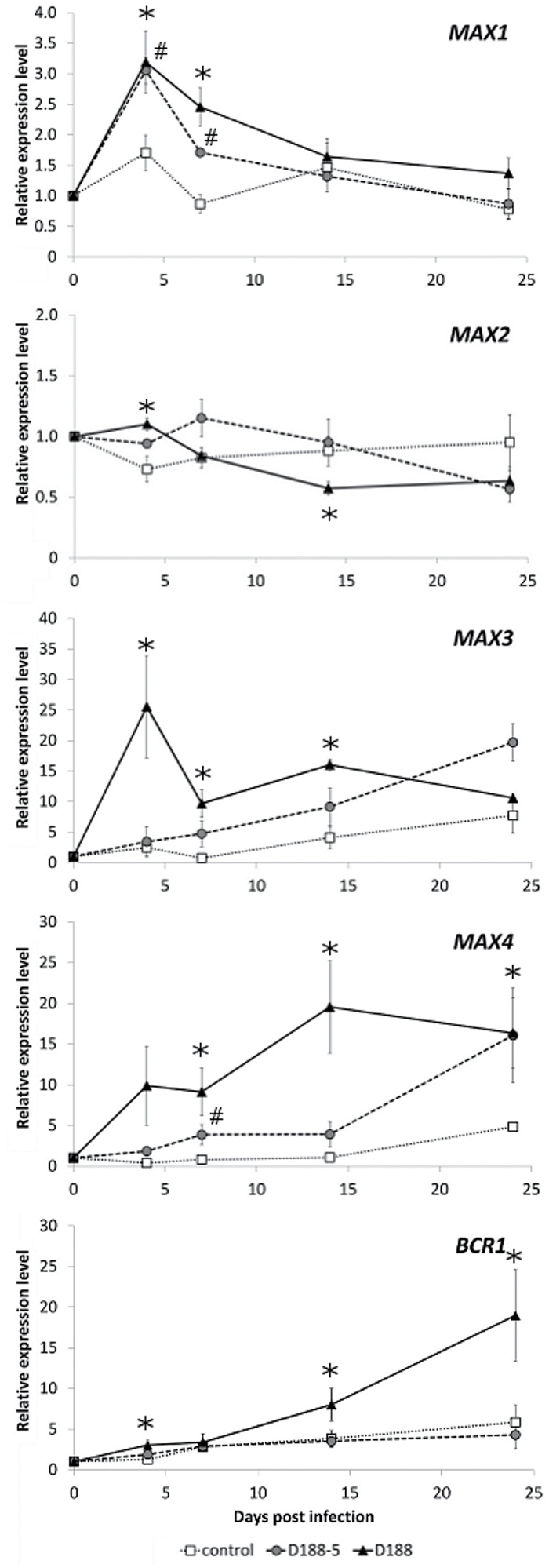
To further investigate the involvement of strigolactones in the pathology induced by R. fascians, the expression profiles of the MAX and BRC1 genes were determined by means of qRT-PCR on shoot tissues sampled at 0, 4, 7, 14, and 24 dpi from Col-0 plants infected with strain D188, and with strain D188-5 or mock-inoculated with water as comparative controls.
In control plants, MAX3, MAX4, and BRC1 expression displayed an upwards trend during plant development, whereas MAX1 and MAX2 expression did not exhibit a clear developmental regulation (Fig. 3). Infection with strain D188-5 had no significant effect on MAX2 and BRC1 expression, but stimulated the transcription of the three strigolactone biosynthesis genes. MAX3 and MAX4 transcript levels gradually increased during the interaction with strain D188-5, reaching 2- and 3-fold higher levels, respectively, than those of the mock-inoculated control at 24 dpi (Fig. 3). In contrast, MAX1 expression was induced transiently up to 2-fold in the first week of the interaction with strain D188-5, but from 14 dpi onwards the transcript level was comparable to that of the control (Fig. 3). Infection with strain D188 provoked a similar expression profile for MAX1, but not for the other genes. Upon D188 infection, MAX3 expression was transiently upregulated 5-fold at 4 dpi and subsequently decreased to a level comparable to that in control plants at 24 dpi. MAX4 expression gradually increased during the interaction with strain D188, but this increase was faster and stronger than upon D188-5 infection until 14 dpi; at 24 dpi, infection with both R. fascians strains resulted in a comparable MAX4 expression level (Fig. 3). MAX2 expression was hardly affected by infection with D188 and did not exceed a 2-fold change (Fig. 3). From 7 days onwards, during the interaction with strain D188, BRC1 transcription was activated to reach a 4-fold higher level than that of the controls at 24 dpi (Fig. 3). Despite the distinct expression patterns of the genes tested, the qRT-PCR analysis of host plant tissues revealed a concerted D188-triggered increase of the expression of the strigolactone biosynthesis genes at the onset of the interaction and a steady induction of the BRC1 gene.
Fig. 3.
Transcript profiles obtained by qRT-PCR of strigolactone-related genes during R. fascians-induced symptom development in Arabidopsis Col-0. Error bars indicate standard errors (n = 3). Hashes and asterisks mark statistically significant differences (Student’s t-test; P < 0.05) between D188-5- and mock-infected (control) samples and between D188-infected and control samples, respectively.
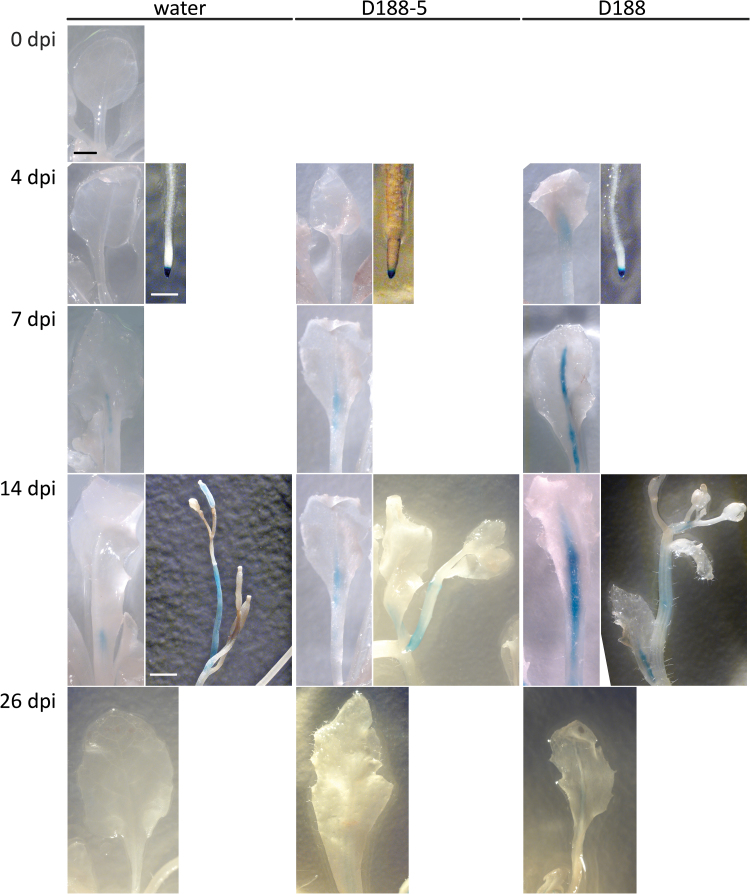
To evaluate the effect of R. fascians infection on the spatial expression pattern of MAX2 and MAX4, 2-week-old Col-0 plants carrying the respective promoter:GUS fusions (Sorefan et al., 2003; Stirnberg et al., 2007) were infected with strains D188-5 or D188 or mock-inoculated with water as a control; the plants were stained histochemically at 4, 7, 14, and 26 dpi. In support of the qRT-PCR data, no differences could be observed at any time point between the three different conditions for the MAX2:GUS line: the expression was strong in the leaves and roots, but was lower in the reproductive tissues (Supplementary Fig. S1) (Stirnberg et al., 2007). By contrast, overall MAX4 expression was weak in the mock-infected plants (Supplementary Fig. S2), except in the root tips where a strong expression was consistently detected (Fig. 4). Moreover, weak expression was occasionally observed in the floral stalks and petioles (Fig. 4), which is in agreement with the expression pattern reported by Sorefan et al. (2003). No ectopic MAX4 expression could be observed upon infection with either of the R. fascians strains (Supplementary Fig. S2), but with D188-5 the expression in the petioles was somewhat stronger than in the mock-infected control, especially at 10 and 14 dpi (Fig. 4). Upon infection with D188, from 4 dpi onward a much stronger expression was detected in the petioles and vasculature of all symptomatic leaves (Fig. 4; Supplementary Fig. S2), supporting the qRT-PCR data. At 26 dpi, hardly any GUS staining could be observed anymore in the aerial parts of all plants tested (Fig. 4, Supplementary Fig. S2)
Fig. 4.
Histochemical analysis of MAX4 expression during R. fascians-induced symptom development in Arabidopsis Col-0. Representative plant parts either mock-inoculated with water or infected with R. fascians strains D188-5 and D188 at different time points. At least 20 plants per time point were infected by placing a 10-µl drop of bacterial suspension at the heart of the rosette. All images for each tissue type were taken at the same magnification. Bar = 1mm.
Thus, in contrast to what might have been predicted from the symptom development on the strigolactone mutants and the pharmacological data, the transcript data show that, upon contact with R. fascians, the strigolactone biosynthesis machinery is activated in the host.
Assessing strigolactone levels in tissues infected with R. fascians
Although it still remains difficult to analyse strigolactones in shoot tissues of Arabidopsis (Seto et al., 2014), the upregulation of the strigolactone biosynthesis genes upon infection with R. fascians was examined at the metabolite level. Shoot material was harvested at 14 dpi and 48 dpi from Col-0 plants and max4 mutants mock-inoculated with water or infected with strain D188. The plant tissues were extracted with ethyl acetate and analysed by LC-MS/MS (see Materials and methods), but strigolactones could not be detected in any of the samples. Because seed germination of the parasitic plant O. minor is strongly stimulated by strigolactones (Goldwasser et al., 2000, 2008), this sensitive bioassay was used to demonstrate the occurrence of strigolactones in tissues infected with D188. As a positive control, O. minor seeds were treated with GR24: at 0.1 µM and 1 µM, an average germination rate was obtained of 11.5% (±5.8% SE) and of 6.5% (±1.8% SE), respectively. Plant tissue extracts were prepared as for the LC-MS/MS analysis, but O. minor seed germination could not be stimulated by these extracts. Finally, preconditioned O. minor seeds were sprinkled on Arabidopsis roots either infected with D188 or mock-inoculated with water (48 dpi), but even after 21 days, no germination occurred. These negative results together with the increased strigolactone biosynthesis suggested by the transcription data indicate that increased strigolactone levels might occur only very localized and/or are very mild.
Bacterial cytokinins trigger the transcriptional strigolactone response through the activation of plant auxin biosynthesis
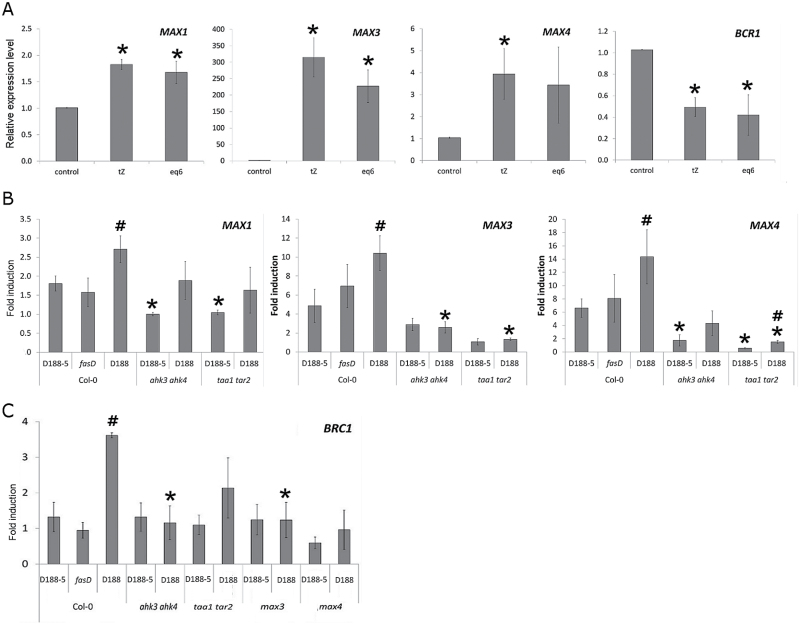
Because cytokinins are the main pathogenicity factor of R. fascians and appear to be at the basis of every response analysed to date (Stes et al., 2011b, 2012, 2013), 2-week-old Col-0 plants were placed on media supplemented either with 10 μM tZ or an equimolar mix of the six cytokinin bases produced by R. fascians (Pertry et al., 2009) and shoot tissues were sampled for molecular profiling 7 days post treatment. Both cytokinin treatments induced MAX1, MAX3, and MAX4 expression (Fig. 5A), implying that the bacterial cytokinins are important signals that can induce strigolactone-associated responses.
Fig. 5.
Importance of cytokinins, auxins, and strigolactones in the strigolactone transcriptional response during the interaction of R. fascians with Arabidopsis. (A) Relative expression levels of strigolactone biosynthesis and BRC1 genes in Col-0 plants treated or not with 10 µM tZ or with an equimolar mix of 1 μM each of 2-iP, tZ, cZ, and their 2MeS derivatives after 7 days of treatment. Error bars are standard errors (n = 3). Asterisks mark statistically significant differences between the fold induction levels in control and cytokinin-treated plants (Student’s t-test; P < 0.05). (B) Fold induction of transcript levels of strigolactone biosynthesis genes upon infection with different R. fascians strains in wild-type, ahk3 ahk4, and taa1 tar2 plants at 7 dpi (R. fascians infection versus mock infection). Error bars are standard errors (n = 3). Hashes and asterisks mark statistically significant differences between the fold induction levels obtained upon infection with the virulence-compromised R. fascians mutants and strain D188, and between wild-type and mutant plants, respectively (Student’s t-test; P < 0.05). (C) Fold induction of BRC1 transcript levels upon infection with different R. fascians strains in wild-type, ahk3 ahk4, taa1 tar2, max3, and max4 plants at 14 dpi (R. fascians infection versus mock infection). Statistics are as in (B).
The impact of the fas-derived cytokinins on strigolactone biosynthetic gene expression was validated by determining the transcription profile of MAX1, MAX3, and MAX4 in Col-0 upon infection with the R. fascians mutant D188-fasD, defective in the isopentenyltransferase and thus impaired in cytokinin production (Pertry et al., 2010). Indeed, the expression level of the strigolactone biosynthesis genes in plants infected by D188-fasD was comparable to that of D188-5-infected plants and significantly lower than that measured upon D188 infection (Fig. 5B). Cytokinin perception by the plant proved to be equally important: in the double cytokinin receptor mutant ahk3 ahk4 (Higuchi et al., 2004), which is not responsive to R. fascians infection (Pertry et al., 2009), MAX3 and MAX4 were no longer induced upon D188 infection, whereas MAX1 activation was reduced (Fig. 5B). The expression level of the two CCD genes in the ahk3 ahk4 mutant infected with either of the R. fascians strains even dropped below that measured in wild-type plants infected with strain D188-5 (Fig. 5B), indicating that expression of these strigolactone biosynthesis genes is highly sensitive, even to low levels of bacterial cytokinins.
In agreement with previous reports (Braun et al., 2012; Dun et al., 2012), BRC1 expression was downregulated by exogenous cytokinins (Fig. 5A). Surprisingly, the induced BRC1 expression seen during leafy gall development (Fig. 3) did not occur upon interaction of Col-0 plants with strain D188-fasD nor in the ahk3 ahk4 mutant infected with strain D188, hinting at a cytokinin-dependent response (Fig. 5C). Based on these observations, the effect of R. fascians on BRC1 expression might be the consequence of the local and/or continuous fas-dependent activation of strigolactone biosynthesis. Indeed, BRC1 expression was not induced in D188-infected max3 and max4 mutants (Fig. 5C).
Because the R. fascians cytokinins target the TAA1 and TAR2 genes of the indole-3-pyruvic acid pathway of Arabidopsis that activates auxin production (Stes et al., 2012) and auxin is a known inducer of MAX3 and MAX4 expression in Arabidopsis (Hayward et al., 2009), the observed cytokinin-dependent increase in MAX gene expression upon R. fascians infection might possibly result from the increased auxin levels in the infected tissues. To investigate this hypothesis, the strigolactone response was analysed in the taa1-1 tar2-1 mutant (Stepanova et al., 2008). This mutant develops fewer symptomatic shoots than the wild-type plants upon D188 infection because plant-derived auxin plays an accessory role in symptom formation (Stes et al., 2012). The upregulation of the strigolactone biosynthesis and BRC1 genes observed in Col-0 plants upon D188 infection did not occur in the taa1-1 tar2-1 mutant (Fig. 5B, C). Hence, the activation of auxin production in the host by the bacterial cytokinins seems to be responsible for the elevated transcription of the tested strigolactone genes in symptomatic tissues.
Discussion
In the last decade, enormous progress has been made in strigolactone research. As a result, multiple roles in plant growth and development have been identified for this class of phytohormones. Moreover, based on accumulating evidence, strigolactones seem to be emerging as integrators of diverse signals involved in abiotic as well as biotic stress-related responses in plants.
Here, the impact of strigolactones was examined in a bacterial pathosystem—the interaction between Arabidopsis and the biotrophic actinomycete R. fascians—in which cytokinins are used as the main pathogenicity factor. Strigolactones were found to play a role as antagonistic compounds that restrict symptom development. Indeed, the tested strigolactone biosynthesis and sensing mutants were hypersensitive to R. fascians and developed stronger symptoms than the wild-type plants, with excessive development of multiple shoots from the axillary meristem regions. Moreover, symptom development was reduced in the presence of exogenous GR24 and stimulated by D2 treatment. Finally, the strigolactone biosynthesis genes were upregulated at the onset (MAX1 and MAX3) and throughout (MAX4) the interaction. The hypersensitivity of the strigolactone-related mutants towards R. fascians infection was not entirely unexpected, because max and brc1 mutants have a broken apical dominance (Stirnberg et al., 2002) and an enhanced sensitivity to cytokinins (Dun et al., 2012).
The expression data point out that strigolactones interfere with the molecular dialogue between Arabidopsis and R. fascians, especially at the onset of the interaction, ultimately constraining to some extent shoot induction and, hence, leafy gall formation. Clearly, activation of the strigolactone biosynthesis genes depends on the bacterial cytokinins that are produced by the main virulence locus fas, because their expression is no longer activated upon infection with the cytokinin-defective mutant strain D188-fasD. The perception of the bacterial cytokinins by the plant receptors AHK3 and AHK4 is equally important for induced strigolactone biosynthetic gene expression. Indeed, upon infection of the non-responsive ahk3 ahk4 mutant with strain D188, MAX3 and MAX4 expression was comparable to that of wild-type plants upon infection with the non-pathogenic strain D188-5. Altogether, these results indicate that the bacterial cytokinins are the main effectors in the strigolactone response of this pathosystem. Nevertheless, the induced expression of MAX3 and MAX4 was also lost in the taa1-1 tar2-1 mutant defective in auxin production, demonstrating that the effect of the bacterial cytokinins is indirect and mediated by host-derived auxin. Interestingly, based on these results, symptom development in the taa1-1 tar2-1 mutant would be expected to be enhanced because of the lack of repressive strigolactones, but this is not the case. In contrast, the taa1-1 tar2-1 mutant has been shown to be less symptomatic than the Col-0 wild type upon D188 infection (Stes et al., 2012). All these data imply that plant-derived auxin plays multiple roles in symptom development: a direct positive effect (Stes et al., 2012) and an indirect negative feedback via strigolactone biosynthesis. Finally, the enhanced strigolactone biosynthetic gene expression in R. fascians-infected tissues and subsequent activation of BRC1 might prevent the outgrowth of the newly developing shoots (Poza-Carrión et al., 2007), which is a hallmark of the leafy gall. Indeed, the BRC1 induction observed in wild-type plants upon infection with strain D188 is lost in the max3 and max4 mutant backgrounds. Despite the clear strigolactone-dependent BRC1 upregulation, strigolactones could not be detected in infected plant tissues, hinting at a very local or mild strigolactone accumulation. Thus, like in most developmental processes in which strigolactones are involved (Cheng et al., 2013), the cross-talk between stri- golactones, cytokinins, and auxins also takes a central position during pathological plant development.
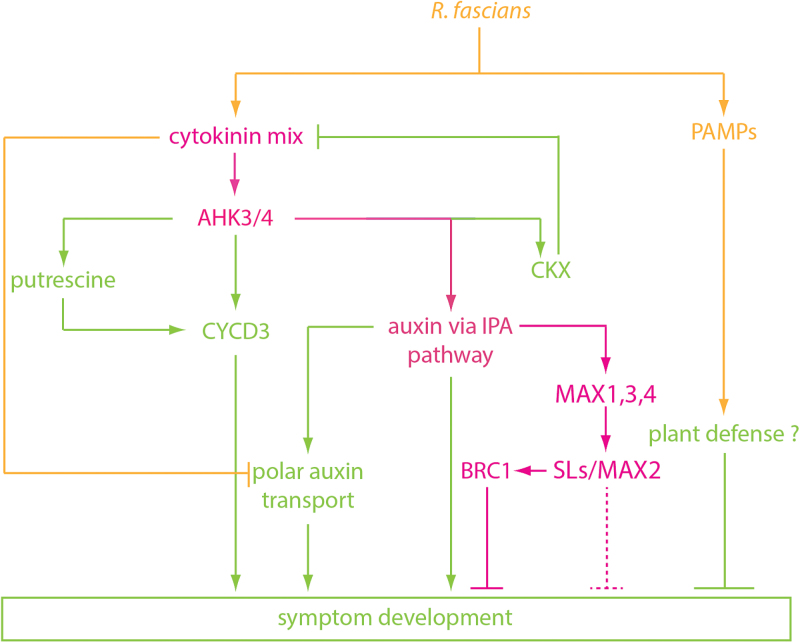
In conclusion, it is becoming increasingly clear that for successful leafy gall formation, balanced responses are critical. Moreover, apparently Arabidopsis defends itself against R. fascians and partially controls the impact of the bacterial cytokinins by acting on the phytohormone level. Based on the data presented, the previously proposed model (Stes et al., 2012) on the molecular basis of leafy gall syndrome can be extended (Fig. 6). Upon perception of the bacterial cytokinin mix by AHK3/AHK4, the plant activates its cytokinin homeostasis mechanisms in a first attempt to counter the morphogenic pressure imposed by R. fascians (Depuydt et al., 2008; Pertry et al., 2009). The bacteria react by triggering the production of auxin and putrescine in the infected plant tissues, which function as accessory signals that aid leafy gall formation (Stes et al., 2011a, 2012). Ultimately, in an effort to further antagonize the action of the R. fascians cytokinins at the target tissues, the plant locally overproduces potent inhibitors of shoot branching, the strigolactones. Through BRC1 the outgrowth of the newly induced shoots is blocked. Because BRC1 is only one of the signalling components in the strigolactone signal transduction pathway, additional direct effects of stri-golactones on symptom formation cannot be ruled out at this moment. The formation of a leafy gall unequivocally signifies that R. fascians wins this hormone battle. Nevertheless, although the typical defence responses generally activated in plants upon perception of pathogen-associated molecular patterns have not been addressed in detail in the R. fascians–Arabidopsis pathosystem, it would be interesting to see whether the basal immunity of the plant exerts some level of control over the pathogen to define the ideal settings for the establishment of a long-lasting biotrophic relation between R. fascians and its host.
Fig. 6.
Extended model of the signalling cascade triggered by the R. fascians cytokinins leading to the strigolactone response (magenta) previous scheme during the R. fascians–Arabidopsis interaction. Orange, bacterial signals; green, plant responses. CKX, cytokinin oxidase/dehydrogenase; CYCD3, D3-type cyclin; IPA, indole pyruvic acid; PAMPs, pathogen-associated molecular patterns; SLs, strigolactones.
Supplementary material
Supplementary material is available at JXB online.
Supplementary Fig. S1. Histochemical analysis of MAX2 expression during R. fascians-induced symptom development on Arabidopsis Col-0.
Supplementary Fig. S2. Histochemical analysis of MAX4 expression during R. fascians-induced symptom development on Arabidopsis Col-0.
Acknowledgements
The authors thank Martine De Cock for help with the preparation of the manuscript. ES and SD are Postdoctoral Fellows of the Research Foundation-Flanders. CM is indebted to the Bijzonder Onderzoeksfonds of Ghent University for a predoctoral fellowship.
Glossary
Abbreviations:
- 2-iP
2-isopentenyladenine
- 2MeS
2-methylthio
- AHK
Arabidopsis histidine kinase
- BRC
BRANCHED
- CCD
carotenoid cleavage dioxygenase
- CKX
CYTOKININ DEHYDROGENASE/OXIDASE
- cZ
cis-zeatin
- D27
DWARF27
- DAD
DECREASED APICAL DOMINANCE
- DMSO
dimethylsulfoxide
- dpi
days post infection
- GR24
strigolactone analogue
- GUS
β-glucuronidase
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- MAX
MORE AXILLARY GROWTH
- qRT-PCR
quantitative reverse-transcription-polymerase chain reaction
- SCF
Skp-Cullin-F-box
- TAA
TRYPTOPHAN AMINOTRANSFERASE
- TAR
TAA1-RELATED
- tZ
trans-zeatin
- WEI
WEAK ETHYLENE INSENSITIVE.
References
- Abe S, Sadob A, Tanaka K, et al. 2014. Carlactone is converted to carlactonoic acid by MAX1 in Arabidopsis and its methyl ester can directly interact with AtD14 in vitro. Proceedings of the National Academy of Sciences, USA 111, 18084–18089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Martínez JA, Poza-Carrión C, Cubas P. 2007. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell 19, 458–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agusti J, Herold S, Schwarz M, et al. 2011. Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proceedings of the National Academy of Sciences, USA 108, 20242–20247 [Erratum, Proceedings of the National Academy of Sciences, USA 109, 14277]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama K, Matsuzaki K-i, Hayashi H. 2005. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435, 824–827. [DOI] [PubMed] [Google Scholar]
- Alder A, Jamil M, Marzorati M, Bruno M, Vermathen M, Bigler P, Ghisla S, Bouwmeester H, Beyer P, Al-Babili S. 2012. The path from β-Carotene to carlactone, a strigolactone-like plant hormone. Science 335, 1348–1351. [DOI] [PubMed] [Google Scholar]
- Besserer A, Puech-Pagès V, Kiefer P, Gomez-Roldan V, Jauneau A, Roy S, Portais J-C, Roux C, Bécard G, Séjalon-Delmas N. 2006. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biology 4, e226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge CA. 2006. Axillary bud outgrowth: sending a message. Current Opinion in Plant Biology 9, 35–40. [DOI] [PubMed] [Google Scholar]
- Bonneau L, Huguet S, Wipf D, Pauly N, Truong H-N. 2013. Combined phosphate and nitrogen limitation generates a nutrient stress transcriptome favorable for arbuscular mycorrhizal symbiosis in Medicago truncatula . New Phytologist 199, 188–202. [DOI] [PubMed] [Google Scholar]
- Booker J, Auldridge M, Wills S, McCarty D, Klee H, Leyser C. 2004. MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Current Biology 14, 1232–1238. [DOI] [PubMed] [Google Scholar]
- Booker J, Sieberer T, Wright W, Williamson L, Willett B, Stirnberg P, Turnbull C, Srinivasan M, Goddard P, Leyser O. 2005. MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Developmental Cell 8, 443–449. [DOI] [PubMed] [Google Scholar]
- Braun N, de Saint Germain A, Pillot J-P, et al. 2012. The pea TCP transcription factor PsBRC1 acts downstream of strigolactones to control shoot branching. Plant Physiology 158, 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer PB, Koltai H, Beveridge CA. 2013. Diverse roles of strigolactones in plant development. Molecular Plant 6, 18–28. [DOI] [PubMed] [Google Scholar]
- Bu Q, Lv T, Shen H, et al. 2014. Regulation of drought tolerance by the F-box protein MAX2 in Arabidopsis . Plant Physiology 164, 424–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Ruyter-Spira C, Bouwmeester H. 2013. The interaction between strigolactones and other plant hormones in the regulation of plant development. Frontiers in Plant Science 4, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier F, Nieminen K, Sánchez-Ferrero JC, Rodríguez ML, Chagoyen M, Hardtke CS, Cubas P. 2014. Strigolactone promotes degradation of DWARF14, an α/β hydrolase essential for strigolactone signaling in Arabidopsis . Plant Cell 26, 1134–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CE, Whichard LP, Turner B, Wall ME. 1966. Germination of witchweed (Striga lutea Lour.): isolation and properties of a potent stimulant. Science 154, 1189–1190. [DOI] [PubMed] [Google Scholar]
- De Cuyper C, Fromentin J, Endah Yocgo R, De Keyser A, Guillotin B, Kunert K, Boyer F-D, Goormachtig S. 2015. From lateral root density to nodule number, the strigolactone analogue GR24 shapes the root architecture of Medicago truncatula . Journal of Experimental Botany 66, 137–146. [DOI] [PubMed] [Google Scholar]
- de O Manes C-L, Van Montagu M, Prinsen E, Goethals K, Holsters M. 2001. De novo cortical cell division triggered by the phytopathogen Rhodococcus fascians in tobacco. Molecular Plant-Microbe Interactions 14, 189–195. [DOI] [PubMed] [Google Scholar]
- de O Manes C-L, Beeckman T, Ritsema T, Van Montagu M, Goethals K, Holsters M. 2004. Phenotypic alterations in Arabidopsis thaliana plants caused by Rhodococcus fascians infection. Journal of Plant Research 117, 139–145. [DOI] [PubMed] [Google Scholar]
- Depuydt S, De Veylder L, Holsters M, Vereecke D. 2009. b . Eternal youth, the fate of developing Arabidopsis leaves upon Rhodococcus fascians infection. Plant Physiology 149, 1387–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depuydt S, Doležal K, Van Lijsebettens M, Moritz T, Holsters M, Vereecke D. 2008. Modulation of the hormone setting by Rhodococcus fascians results in ectopic KNOX activation in Arabidopsis . Plant Physiology 146, 1267–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depuydt S, Trenkamp S, Fernie AR, Elftieh S, Renou J-P, Vuylsteke M, Holsters M, Vereecke D. 2009. a . An integrated genomics approach to define niche establishment by Rhodococcus fascians . Plant Physiology 149, 1366–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desomer J, Dhaese P, Van Montagu M. 1988. Conjugative transfer of cadmium resistance plasmids in Rhodococcus fascians strains. Journal of Bacteriology 170, 2401–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domagalska MA, Leyser O. 2011. Signal integration in the control of shoot branching. Nature Reviews Molecular Cell Biology 12, 211–221. [DOI] [PubMed] [Google Scholar]
- Dor E, Joel DM, Kapulnik Y, Koltai H, Hershenhorn J. 2011. The synthetic strigolactone GR24 influences the growth pattern of phytopathogenic fungi. Planta 234, 419–427. [DOI] [PubMed] [Google Scholar]
- Dun EA, de Saint Germain A, Rameau C, Beveridge CA. 2012. Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiology 158, 487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo E, Ferguson BJ, Reid JB. 2014. The potential roles of strigolactones and brassinosteroids in the autoregulation of nodulation pathway. Annals of Botany 113, 1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis I, De Keyser A, De Backer P, et al. 2012. pFiD188, the linear virulence plasmid of Rhodococcus fascians strain D188. Molecular Plant-Microbe Interactions 25, 637–647. [DOI] [PubMed] [Google Scholar]
- Goldwasser Y, Plakhine D, Yoder JI. 2000. Arabidopsis thaliana susceptibility to Orobanche spp. Weed Science 48, 342–346. [Google Scholar]
- Goldwasser Y, Yoneyama K, Xie X, Yoneyama K. 2008. Production of strigolactones by Arabidopsis thaliana responsible for Orobanche aegyptiaca seed germination. Plant Growth Regulation 55, 21–28. [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, et al. 2008. Strigolactone inhibition of shoot branching. Nature 455, 189–194. [DOI] [PubMed] [Google Scholar]
- González-Pérez S, Gutiérrez J, García–García F, Osuna D, Dopazo J, Lorenzo Ó, Revuelta JL, Arellano JB. 2011. Early transcriptional defense responses in Arabidopsis cell suspension culture under high-light conditions. Plant Physiology 156, 1439–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha CV, Leyva-González MA, Osakabe Y, et al. 2014. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proceedings of the National Academy of Sciences, USA 111, 851–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamiaux C, Drummond RSM, Janssen BJ, Ledger SE, Cooney JM, Newcomb RD, Snowden KC. 2012. DAD2 is an a/β hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Current Biology 22, 2032–2036. [DOI] [PubMed] [Google Scholar]
- Hayward A, Stirnberg P, Beveridge C, Leyser O. 2009. Interactions between auxin and strigolactone in shoot branching control. Plant Physiology 151, 400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Pischke MS, Mähönen AP, et al. 2004. In planta functions of the Arabidopsis cytokinin receptor family. Proceedings of the National Academy of Sciences, USA 101, 8821–8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa S, Maekawa M, Arite T, Onishi K, Takamure I, Kyozuka J. 2005. Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant and Cell Physiology 46, 79–86. [DOI] [PubMed] [Google Scholar]
- Jia K-P, Luo Q, He S-B, Lu X-D, Yang H-Q. 2014. Strigolactone-regulated hypocotyl elongation is dependent on cryptochrome and phytochrome signaling pathways in Arabidopsis . Molecular Plant 7, 528–540. [DOI] [PubMed] [Google Scholar]
- Jiang L, Liu X, Xiong G, et al. 2013. DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature 504, 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson X, Brcich T, Dun EA, Goussot M, Haurogné K, Beveridge CA, Rameau C. 2006. Branching genes are conserved across species. Genes controlling a novel signal in pea are coregulated by other long-distance signals. Plant Physiology 142, 1014–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapulnik Y, Delaux P-M, Resnick N, et al. 2011. Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis . Planta 233, 209–216. [DOI] [PubMed] [Google Scholar]
- Kohlen W, Charnikhova T, Liu Q, Bours R, Domagalska MA, Beguerie S, Verstappen F, Leyser O, Bouwmeester H, Ruyter-Spira C. 2011. Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis . Plant Physiology 155, 974–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Novero M, Charnikhova T, Ferrandino A, Schubert A, Ruyter-Spira C, Bonfante P, Lovisolo C, Bouwmeester HJ, Cardinale F. 2013. CAROTENOID CLEAVAGE DIOXYGENASE 7 modulates plant growth, reproduction, senescence, and determinate nodulation in the model legume Lotus japonicus . Journal of Experimental Botany 64, 1967–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzec M, Muszynska A, Gruszka D. 2013. The role of strigolactones in nutrient-stress responses in plants. International Journal of Molecular Sciences 14, 9286–9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matusova R, Rani K, Verstappen FWA, Franssen MCR, Beale MH, Bouwmeester HJ. 2005. The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiology 139, 920–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Xue Y-L, Miyakawa T, et al. 2013. Molecular mechanism of strigolactone perception by DWARF14. Nature Communications 4, 2613. [DOI] [PubMed] [Google Scholar]
- Pertry I, Václavíková K, Depuydt S, et al. 2009. Identification of Rhodococcus fascians cytokinins and their modus operandi to reshape the plant. Proceedings of the National Academy of Sciences, USA 106, 929–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertry I, Václavíková K, Gemrotová M, et al. 2010. Rhodococcus fascians impacts plant development through the dynamic Fas-mediated production of a cytokinin mix. Molecular Plant-Microbe Interactions 23, 1164–1174. [DOI] [PubMed] [Google Scholar]
- Poza-Carrión C, Aguilar-Martínez JA, Cubas P. 2007. Role of TCP gene BRANCHED1 in the control of shoot branching in Arabidopsis . Plant Signaling & Behavior 2, 551–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen A, Mason MG, De Cuyper C, Brewer PB, Herold S, Agusti J, Geelen D, Greb T, Goormachtig S, Beeckman T, Beveridge CA. 2012. Strigolactones suppress adventitious rooting in Arabidopsis and pea. Plant Physiology 158, 1976–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyter-Spira C, Kohlen W, Charnikhova T, et al. 2011. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: another belowground role for strigolactones? Plant Physiology 155, 721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K, Yoneyama K, Kondoh H, Shimizu T, Sadaya T, Choi I-R, Yoneyama K, Omura T, Kikuchi S. 2013. Relationship between gene responses and symptoms induced by Rice grassy stunt virus. Frontiers in Microbiology 4, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi A, Waters MT, Sun YK, Skelton BW, Dixon KW, Ghisalberti EL, Flematti GR, Smith SM. 2014. Strigolactone hormones and their stereoisomers signal through two related receptor proteins to induce different physiological responses in Arabidopsis . Plant Physiology 165, 1221–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant MJ, Li J-J, Fox C, Brookbank N, Rea D, Bugg TDH, Thompson AJ. 2009. Selective inhibition of carotenoid cleavage dioxygenases. Phenotypic effects on shoot branching. Journal of Biological Chemistry 284, 5257–5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto Y, Sado A, Asami K, Hanada A, Umehara M, Akiyama K, Yamaguchi S. 2014. Carlactone is an endogenous biosynthetic precursor for strigolactones. Proceedings of the National Academy of Sciences, USA 111, 1640–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Luong P, Huq E. 2007. The F-box protein MAX2 functions as a positive regulator of photomorphogenesis in Arabidopsis . Plant Physiology 145, 1471–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Zhu L, Bu Q-Y, Huq E. 2012. MAX2 affects multiple hormones to promote photomorphogenesis. Molecular Plant 5, 750–762. [DOI] [PubMed] [Google Scholar]
- Simón-Mateo C, Depuydt S, de Oliveira Manes CL, Cnudde F, Holsters M, Goethals K, Vereecke D. 2006. The phytopathogen Rhodococcus fascians breaks apical dominance and activates axillary meristems by inducing plant genes involved in hormone metabolism. Molecular Plant Pathology 7, 103–112. [DOI] [PubMed] [Google Scholar]
- Snowden KC, Simkin AJ, Janssen BJ, Templeton KR, Loucas HM, Simons JL, Karunairetnam S, Gleave AP, Clark DG, Klee HJ. 2005. The Decreased apical dominance1/Petunia hybrida CAROTENOID CLEAVAGE DIOXYGENASE8 gene affects branch production and plays a role in leaf senescence, root growth, and flower development. Plant Cell 17, 746–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorefan K, Booker J, Haurogné K, et al. 2003. MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes & Development 17, 1469–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto MJ, Fernández-Aparicio M, Castellanos-Morales V, García-Garrido JM, Ocampo JA, Delgado MJ, Vierheilig H. 2010. First indications for the involvement of strigolactones on nodule formation in alfalfa (Medicago sativa). Soil Biology & Biochemistry 42, 383–385. [Google Scholar]
- Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie D-Y, Doležal K, Schlereth A, Jürgens G, Alonso JM. 2008. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133, 177–191. [DOI] [PubMed] [Google Scholar]
- Stes E, Biondi S, Holsters M, Vereecke D. 2011. a . Bacterial and plant signal integration via D3-type cyclins enhances symptom development in the Arabidopsis-Rhodococcus fascians interaction. Plant Physiology 156, 712–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stes E, Francis I, Pertry I, Dolzblasz A, Depuydt S, Vereecke D. 2013. The leafy gall syndrome induced by Rhodococcus fascians . FEMS Microbiology Letters 342, 187–194. [DOI] [PubMed] [Google Scholar]
- Stes E, Prinsen E, Holsters M, Vereecke D. 2012. Plant-derived auxin plays an accessory role in symptom development upon Rhodococcus fascians infection. Plant Journal 70, 513–527. [DOI] [PubMed] [Google Scholar]
- Stes E, Vandeputte O, El Jaziri M, Holsters M, Vereecke D. 2011. b . A successful bacterial coup d’état: how Rhodococcus fascians redirects plant development. Annual Review of Phytopathology 49, 69–86. [DOI] [PubMed] [Google Scholar]
- Stirnberg P, Furner IJ, Leyser HMO. 2007. MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. Plant Journal 50, 80–94. [DOI] [PubMed] [Google Scholar]
- Stirnberg P, van de Sande K, Leyser HMO. 2002. MAX1 and MAX2 control shoot lateral branching in Arabidopsis . Development 129, 1131–1141. [DOI] [PubMed] [Google Scholar]
- Torres-Vera R, García JM, Pozo MJ, López-Ráez JA. 2013. Do strigolactones contribute to plant defence? Molecular Plant Pathology 15, 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Yoshida S, et al. 2008. Inhibition of shoot branching by new terpenoid plant hormones. Nature 455, 195–200. [DOI] [PubMed] [Google Scholar]
- Waldie T, McCulloch H, Leyser O. 2014. Strigolactones and the control of plant development: lessons from shoot branching. Plant Journal 79, 607–622. [DOI] [PubMed] [Google Scholar]
- Wang Y, Sun S, Zhu W, Jia K, Yang H, Wang X. 2013. Strigolactone/MAX2-induced degradation of brassinosteroid transcriptional effector BES1 regulates shoot branching. Developmental Cell 27, 681–688. [DOI] [PubMed] [Google Scholar]
- Waters MT, Nelson DC, Scaffidi A, Flematti GR, Sun YK, Dixon KW, Smith SM. 2012. Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis . Development 139, 1285–1295. [DOI] [PubMed] [Google Scholar]
- Xie X, Yoneyama K, Yoneyama K. 2010. The strigolactone story. Annual Review of Phytopathology 48, 93–117. [DOI] [PubMed] [Google Scholar]
- Yoneyama K, Yoneyama K, Takeuchi Y, Sekimoto H. 2007. Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta 225, 1031–1038. [DOI] [PubMed] [Google Scholar]
- Zhou F, Lin Q, Zhu L, et al. 2013. D14--SCFD3-dependent degradation of D53 regulates strigolactone signalling. Nature 504, 406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.