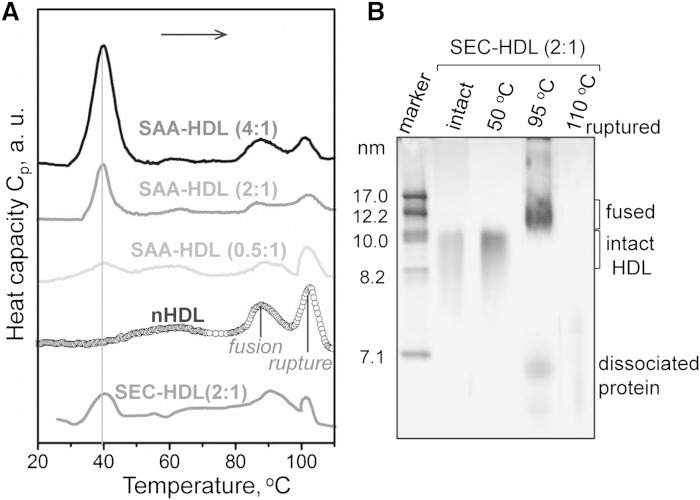
Fig. 4.
Thermal denaturation of SAA-HDL analyzed by DSC and NDGE. A: DSC data of SAA-HDL were recorded during heating from 5°C to 120°C at a rate of 90°C/h. The samples contained 0.8 mg/ml apoA-I and various amounts of SAA at indicated SAA:apoA-I molar ratios. SEC-HDL is the lipoprotein-only fraction isolated by SEC from the total SAA-HDL (2:1 SAA:apoA-I). The data are shifted along the y-axis to avoid overlap. B: NDGE of SEC-HDLs that were intact or heated to 50, 95, or 110°C. Heating to 50°C does not cause HDL remodeling. Heating to 95°C causes partial protein dissociation and HDL fusion; Western blotting detects SAA and apoA-I both in fused HDL and in the dissociated protein (data not shown). Heating to 110°C causes lipoprotein disruption and coalescence into protein-containing lipid droplets that do not enter the gel. For more detailed biochemical analysis of the products of HDL thermal denaturation, see (59).