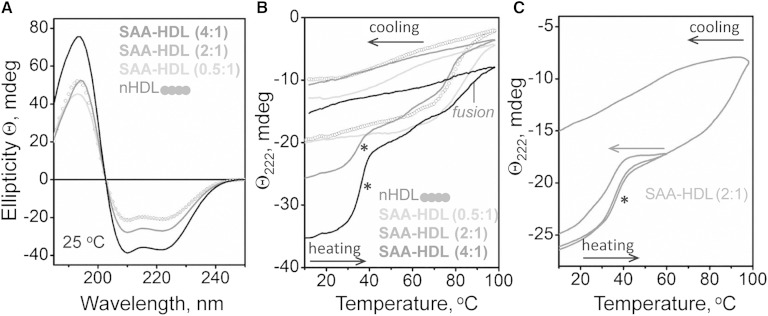
Fig. 5.
Analysis of the heat-induced secondary structural changes in SAA-HDL by far-UV CD. HDLs (20 μg/ml protein in standard buffer) have been incubated at indicated SAA:apoA-I molar ratios to generate SAA-HDL. The samples were placed in a 5 mm path length cell; the data were not normalized to protein concentration. A: Far-UV CD spectra of SAA-HDL at 25°C. B: Heating and cooling data, Θ222(T), recorded at 222 nm at a scan rate of 6°C/h. Arrows show direction of temperature changes; asterisk indicates the unfolding transition at near-physiological temperatures. C: CD melting data recorded from two identical samples of SAA-HDL (2:1 SAA:apoA-I), one heated and cooled to 60°C and another to 98°C. The heating rate was 6°C/h.