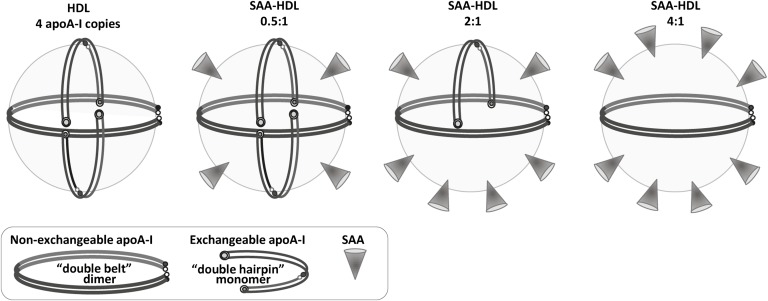
Fig. 8.
Hypothetical structural model of mature plasma HDL containing increasing amounts of SAA. The model that exemplifies nHDL shows four copies of apoA-I, including one molecular dimer in a double-belt conformation and two monomers in a putative double-hairpin conformation. For details of these conformations and alternative models, see (69) and references therein. The SAA:apoA-I molar ratios are indicated. Addition of SAA initially leads to its binding to the available HDL surface (0.5:1 SAA:apoA-I mol/mol), followed by progressive displacement of the exchangeable fraction of apoA-I. We propose that this readily exchangeable apoA-I is probably monomeric, while the less exchangeable apoA-I that determines HDL stability is dimeric. HDL incubation at 4:1 molar (or 1.7:1 weight) ratio of SAA:apoA-I, which displaces approximately two-thirds of apoA-I from HDL (Fig. 1), must be compensated by binding of at least an equivalent amount of SAA in order to maintain the particle density in the HDL3 range. Thus, the HDL-bound proteins comprise roughly two-thirds SAA and one-third apoA-I by weight, which resembles their ratio in the incubation mixture. Such a ratio of bound SAA to apoA-I can be achieved if HDL contains 2 apoA-I molecules and 8 to 10 SAA molecules (right panel).