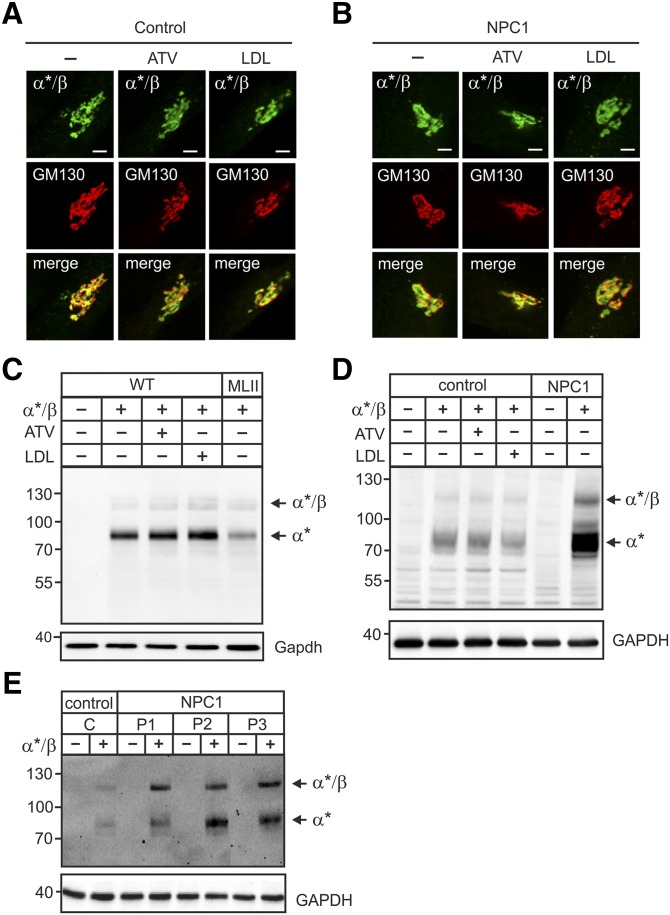
Fig. 3.
ATV or LDL overload did not affect the localization or proteolytic cleavage of the GlcNAc-1-phosphotransferase α*/β-subunit precursor protein. A–E: Human fibroblasts of healthy controls and NPC1 patients, WT and MLII MEFs were cultured in DMEM containing 10% NBCS or 10% LPDS supplemented with 10 µM ATV or 100 µg/ml human LDL for 24 h as indicated. Cells were then transfected with cDNA of α*/β-subunit precursor construct and analyzed after 24 h. A and B: The localization of α*/β-subunit precursor of the GlcNAc-1-phosphotransferase (α*/β, green) was determined in control patient (A) and in NPC1 patient 1 (P1) (B) fibroblasts by costaining with the cis-Golgi marker protein GM130 (red) using immunofluorescence microscopy. Colocalization in merged images appears yellow. Scale bars, 5 µm. C and D: The proteolytic cleavage of the α*/β-subunit precursor as indicator for correct transport to the Golgi apparatus was analyzed by α-subunit Western blot of transfected WT and MLII MEFs (C), human control and NPC1 (P1) fibroblasts (D), and three different NPC1 cell lines (P1, P2 and P3) (E). GAPDH/GAPDH Western blot analysis was used as a loading control. Extracts of nontransfected cells were used as a negative control. The positions of molecular mass marker proteins (in kDa), α*/β-subunit precursor, and mature α*-subunit are indicated.