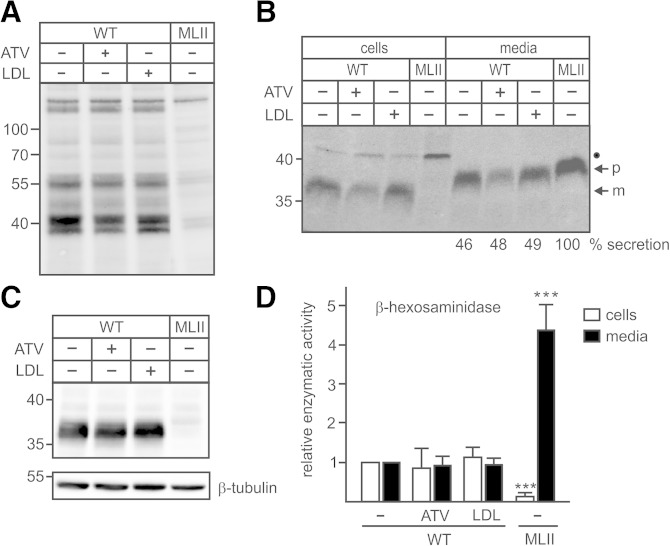
Fig. 4.
ATV or LDL overload did not affect GlcNAc-1-phosphotransferase activity and transport of lysosomal enzymes. A–D: WT and MLII MEFs were cultured in DMEM containing 10% NBCS or 10% LPDS supplemented with 10 µM ATV or 100 µg/ml human LDL for 48 h. A: The GlcNAc-1-phosphotransferase activity in cell extracts was indirectly determined by M6P Western blotting. The positions of the molecular mass markers (in kDa) are indicated. B: The biosynthesis and sorting of the lysosomal protease CtsZ was analyzed by labeling of MEFs with [35S]methionine for 1 h followed by 4 h incubation in nonradioactive medium and immunoprecipitation of CtsZ from cell extracts and media, SDS-PAGE, and fluorography. The amounts of secreted CtsZ precursors were estimated by densitometry and expressed as percentage of totally synthesized CtsZ. The positions of the molecular mass marker proteins (in kDa), precursor (p), and mature (m) form of CtsZ are indicated. The identity of the 45 kDa 35S-labeled polypeptide immunoprecipitated from cell extracts (closed circle) is unknown. C: The total intracellular CtsZ expression at steady state was analyzed by Western blotting. Anti β-tubulin Western blot was used as a loading control. The positions of the molecular mass markers (in kDa) are indicated. D: The relative enzyme activity of the lysosomal enzyme β-hexosaminidase was measured in cell extracts and media conditioned for 24 h. The activities in control WT MEFs incubated with DMEM containing 10% NBCS were set at 1. The specific activity of β-hexosaminidase in these cells and media were 57.7 mU/h/mg protein ± 14.0 and 4.1 mU/24 h/mg protein ± 1.3, respectively. *** P ≤ 0.005.