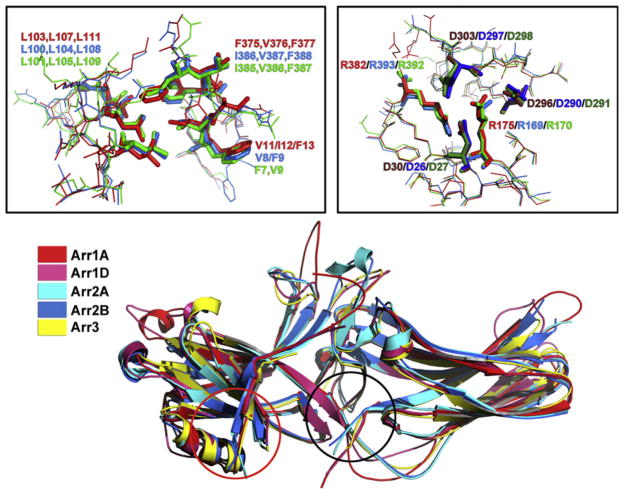
Figure 3.1.
Basal conformation of different arrestin subtypes. Superimposition of the crystal structures of the two monomers in the arrestin-1 tetramer (Arr1A, red and Arr1D, pink),18 two monomers of the arrestin-2 dimer (Arr2A, light blue and Arr2B, dark blue),19 and arrestin-3 (Arr3, yellow)20 shows remarkably similar cores of both domains and variable structure of the loops and inter-domain hinge. Importantly, the variability of these elements in different monomers of the same arrestin (compare Arr1A and Arr1D, as well as Arr2A and Arr2B) is essentially as great as between arrestin subtypes, suggesting that it reflects the flexibility of these loops, rather than their subtype-specific conformations. Black and red circles show the location of the two key intramolecular interactions that hold arrestins in their basal state, the polar core in the inter-domain interface, and the three-element interaction between β-strand I, α-helix I, and β-strand XX in the C-tail, respectively. The panels above show detailed structure of these elements revealing their extremely high conservation, down to the orientation of the side chains. Right: The polar core, main phosphate sensor. Left: The three-element interaction. In both panels, residue numbers of bovine proteins are indicated as follows: arrestin-1, red; arrestin-2, blue; arrestin-3, green.