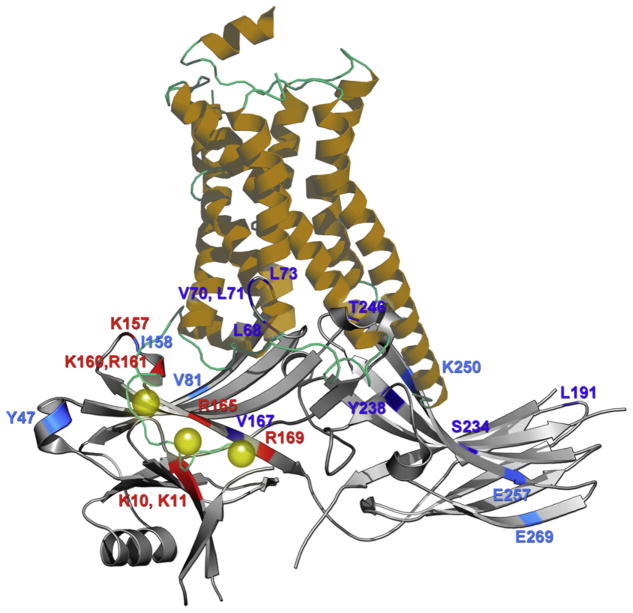
Figure 3.2.
Comparison of the cytoplasmic side of active receptor and arrestin. Putative complex assembled from crystal structure of active β2-adrenergic receptor (from the complex with Gs heterotrimer44) and arrestin-2.19 Phosphate-binding residues and other elements that likely come into direct contact with receptor are shown in red and blue, respectively. Darker blue shows residues in positions where the mobility of the engineered spin label is dramatically decreased upon receptor binding, whereas lighter blue denotes smaller decreases in spin label mobility (based on Ref. 71). Residue numbers correspond to bovine arrestin-2 used in.71 The comparison of these structures suggests that the receptor-binding surface of inactive arrestin-2 is greater than the cytoplasmic part of the receptor resolved in crystal. The receptor C-terminus (not resolved in any crystal structure) with attached phosphates (yellow spheres) was added manually to position the phosphates near known phosphate-binding positive charges in arrestin. The analysis of receptor-binding-induced conformational changes in arrestin-172 revealed very small shifts in relative positions of the two arrestin domains, moderate movement of the “finger loop” toward the receptor, large movement of the neighboring “139 loop” toward the N-domain and to the side (out of the way of incoming receptor), as well as the movement of two loops at the distal tips of the N- and C-domain toward the receptor. Collectively, these rearrangements would allow the finger loop to insert itself deep into the cavity between receptor helices that opens upon activation, and move the tips of the arrestin domains closer to the receptor. However, all contacts expected based on EPR studies of binding-induced changes of spin label mobility in arrestin-173 and -271 can only be readily achieved if the receptor helices move even further apart than they do in complex with Gs.44