Abstract
Inadvertent vaccine freezing often occurs in the cold chain and may cause damage to freeze‑sensitive vaccines. Liquid vaccines that contain aluminum salt adjuvants are particularly vulnerable. Polyol cryoprotective excipients have been shown to prevent freeze damage to hepatitis B vaccine. In this study, we examined the freeze-protective effect of propylene glycol on diphtheria-tetanus-pertussis-whole-cell (DTwP) and acellular (DTaP) vaccines. Pilot lots of DTwP and DTaP formulated with 7.5% propylene glycol underwent 3 freeze-thaw treatments. The addition of propylene glycol had no impact on pH, particle size distribution, or potency of the vaccines prior to freeze-thaw treatment; the only change noted was an increase in osmolality. The potencies and the physical properties of the vaccines containing cryoprotectant were maintained after freeze-thawing and for 3 months in accelerated stability studies. The results from this study indicate that formulating vaccines with propylene glycol can protect diphtheria-tetanus-pertussis vaccines against freeze damages.
Keywords: cold chain, formulation, potency, protection, stability, vaccine freezing
All vaccines lose potency over time. Because the rate of loss is temperature dependent, vaccines are stored and transported in a cold chain. For some vaccines, especially those formulated with an aluminum salt adjuvant, freezing temperatures can be more damaging than exposure to warmer temperatures outside the cold chain. Freeze-thawing causes the agglomeration and sedimentation of antigen-adjuvant particles, resulting in irreversible potency loss.
Vaccines often are exposed unintentionally to freezing temperatures in the cold chain or during distribution and delivery in cold environments.1 Vaccine freezing can lead to wastage (when vaccines are discarded due to suspected damage) or the inadvertent administration of subpotent vaccine, which increases the risk that patients are not fully protected from disease. Freeze-damaged vaccines can be detected using the “shake test,”2,3 but the test is not always performed correctly and does not prevent wastage of damaged vaccines.4,5 An alternative approach is to formulate vaccines so that they are not damaged by freezing temperatures. Previous PATH studies show that the addition of excipients such as glycerin, polyethylene glycol 300, or propylene glycol (PG) can greatly reduce the freeze sensitivity of hepatitis B vaccine.6,7 We have now extended these studies to examine the cryoprotective effect of PG on diphtheria-tetanus-pertussis vaccines.
Preliminary in vitro experiments had indicated that DTaP vaccines were sensitive to freeze‑thawing and that high concentrations of PG (30% v/v) protected them from damage.6 However, these data used particle size as the only readout and evaluated only diphtheria-tetanus-acellular-pertussis (DTaP), whereas diphtheria-tetanus-pertussis-whole-cell (DTwP)-containing vaccines are used more widely in resource-poor settings where temperature excursions in the cold-chain might be more frequent. Since it is important to keep the excipient to the minimal concentration in a vaccine, in this study we also investigated the protective effect of 7.5% (v/v) PG on DTwP and DTaP using standardized potency assays. Further, the vaccine formulations used in this study were prepared by following Good Manufacturing Practices and the stability studies were conducted in compliances with regulatory requirements.
Two 10-liter batches of each of DTwP and DTaP vaccines were prepared for this study by the Wuhan Institute of Biological Products. The compositions of the vaccines were:
DTwP: diphtheria toxoid, 20 Lf (flocculation units)/mL; tetanus toxoid, 5 Lf/mL; whole cell pertussis, 35 x 108 cells/mL; aluminum hydroxide, 1.2 mg/mL. The final pH was 6.0.
DTaP: diphtheria toxoid, 25 Lf/mL; tetanus toxoid, 7 Lf/mL; acellular pertussis, 18 μg protein/mL; aluminum hydroxide, 1.2 mg/mL. The final pH was 6.1. The acellular pertussis antigens were pertussis toxin and filamentous haemagglutinin.8
The minimum required potency for each of the components per 0.5-mL dose was 4 IU for whole cell pertussis or acellular pertussis, 30 IU for diphtheria, and 60 IU for tetanus toxoid. PG (Sinopharm Chemical Reagent Co, Ltd, Shanghai, China) was added to one batch of each of the vaccines prior to final pH adjustment. The final concentration of PG in the vaccine was 7.5% (v/v).
Some vaccines were exposed to freeze-thaw treatment (FT). A “freeze-thaw treatment” consisted of 3 cycles of freezing and thawing. Each cycle consisted of freezing at −20° C for 20 hours, followed by thawing at 22°–25°C for 4 hours. After treatment, vaccines were stored at either 2°–8°C or 22°–25°C (humidity 45%–60%) for 0, 1, or 3 months.
All formulations were analyzed for their physical properties. The results indicated that PG had no effect on the pH of the formulations or on the adsorption of the antigens to the adjuvant under all conditions (data not shown). The only change was that addition of PG (7.5% v/v) to DTwP and DTaP increased the osmolality from 257 and 325 mOsmol/kg to 1,407 and 1,538 mOsmol/kg, respectively. The osmolality was not affected by freeze-thaw treatment or by storage for up to 3 months at either 2°–8°C or 22°–25°C (data not shown).
A potential concern of high osmolality is that the vaccine may cause transient injection site pain. Previous PATH studies demonstrated that hepatitis B vaccine with up to 15% PG was well tolerated by rabbits.7 A study in adults on the impact of osmolality on pain and burning sensations felt following intramuscular injection of 0.5 ml found no dose-effect relationship between these sensations and osmolality. In this study, solutions up to 1,100 mOsmol/kg in humans were tested and were well tolerated.10 Further, Bacillus Calmette–Guérin vaccine and vaccines for subcutaneous allergy immunotherapy are well tolerated even though these vaccines have very high osmolarity. While this evidence seems to suggest the DTP vaccines containing 7.5% PG should be well tolerated, clinical studies will be needed.
Previous studies have shown that freeze-damage to a vaccine is associated with increased particle sizes of the aluminum adjuvant.6 We therefore analyzed the particle size distribution for all samples from this study. The median particles size (D50) in untreated vaccine formulations at the zero time point was unaffected by addition of PG (Table 1). The distribution of particle sizes (D10, D90) was also unchanged by addition of PG (Table 1). Freeze-thaw treatment of formulations without PG resulted in an approximate doubling of median particle size at time zero, indicating agglomeration. Freeze-thawing of DTwP in the absence of PG also resulted in an approximate doubling of D10, and a 75% increase in D90. These increases were not seen in the presence of PG. There was a wider distribution of particle sizes in the DTaP samples before freeze-thawing. Freeze-thaw treatment of DTaP without PG resulted in an increase in the median particle size and D10, but not D90, suggesting agglomeration of the smaller particles in the sample.
Table 1.
Particle size distribution in DTwP and DTaP vaccines formulated with or without 7.5% (v/v) propylene glycol (PG)
| Time points (months) | Particle size (μm) distribution: D50 (D10, D90)* | |||
|---|---|---|---|---|
| No FT, 2–8°C | No FT, 22–25°C | FT, 2–8°C | FT,22–25°C | |
| DTwP, no PG | ||||
| 0 | 15.4 (4.6, 50.2) | † | 33.1 (9.8, 85.1) | † |
| 1 | 15.3 (4.6, 49.0) | 12.5 (4.0, 41.1) | 31.4 (9.7, 76.2) | 29.2 (9.1, 65.7) |
| 3 | 14.9 (4.9, 45.1) | 13.7 (4.4, 39.8) | 25.6 (8.2, 58.4) | 27.9 (8.1, 69.1) |
| DTwP+7.5% PG | ||||
| 0 | 15.8 (4.7, 61.4) | † | 15.8 (5.1, 46.2) | † |
| 1 | 14.6 (4.4, 54.5) | 15.5 (4.7, 57.4) | 12.3 (4.5, 45.3) | 14.6 (5.1, 37.6) |
| 3 | 15.9 (4.7, 56.5) | 14.4 (4.2, 51.5) | 14.4 (4.5, 48.2) | 15.3 (4.9, 60.4) |
| DTaP, no PG | ||||
| 0 | 10.9 (4.2, 94.3) | † | 28.2 (9.4, 78.0) | † |
| 1 | 10.0 (3.5, 157.2) | 10.4 (3.8, 98.8) | 26.6 (9.4, 69.4) | 26.8 (8.8, 65.7) |
| 3 | 10.8 (4.6, 86.2) | 8.2 (3.3, 66.3) | 24.3 (8.7, 59.8) | 24.3 (8.7, 55.0) |
| DTwP+7.5% PG | ||||
| 0 | 12.1 (5.1, 110.5) | † | 10.2 (3.9, 78.5) | † |
| 1 | 11.8 (4.7, 135.5) | 10.7 (4.0, 131.2) | 10.7 (3.7, 82.0) | 13.9 (6.4, 48.4) |
| 3 | 10.1 (4.1, 100.8) | 13.8 (6.2, 104.7) | 10.8 (4.0, 97.3) | 12.7 (5.8, 49.4) |
The vaccines were exposed to freeze-thaw treatment (FT) or not exposed (no FT). A “freeze-thaw treatment” consisted of 3 cycles of freezing and thawing. Each cycle consisted of freezing at -20°C for 20 hours, followed by thawing at 22°–25°C for 4 hours. After treatment, vaccines were stored at either 2°–8°C or 22°–25°C (humidity 45%–60%) for 0, 1, or 3 months. Particle size was measured using a Malvern Mastersizer 2000 (Malvern Instruments, United Kingdom).
* The median particle size, D50, is defined as the diameter at which 50% of a particle's mass is comprised of smaller particles. Particle sizes D10 and D90 are respectively associated with 10% and 90% of the distributed population.
† At 0 month, particle size samples at 22°–25°C were equivalent to samples at 2°–8°C.
There was no further increase in size following 3 months of storage. The presence of 7.5% PG completely protected DTwP and DTaP formulations from agglomeration following freeze-thaw treatment and for up to 3 months at 2°–8°C or 22°–25°C.
The observation that PG prevented agglomeration of the vaccines caused by freeze-thawing suggested that the immunogenicity of the vaccines might have been preserved. This was examined further by testing whether PG could prevent a loss of vaccine potency caused by freeze-thawing of the vaccines. Preliminary studies had shown that diphtheria toxoid and tetanus toxoid are more resistant to freeze damage and more stable at elevated temperatures than pertussis antigens (data not shown). Therefore, for the current study, we focused on testing PG for prevention of freeze damage to the pertussis antigens in DTwP and DTaP vaccines to minimize the use of animals.
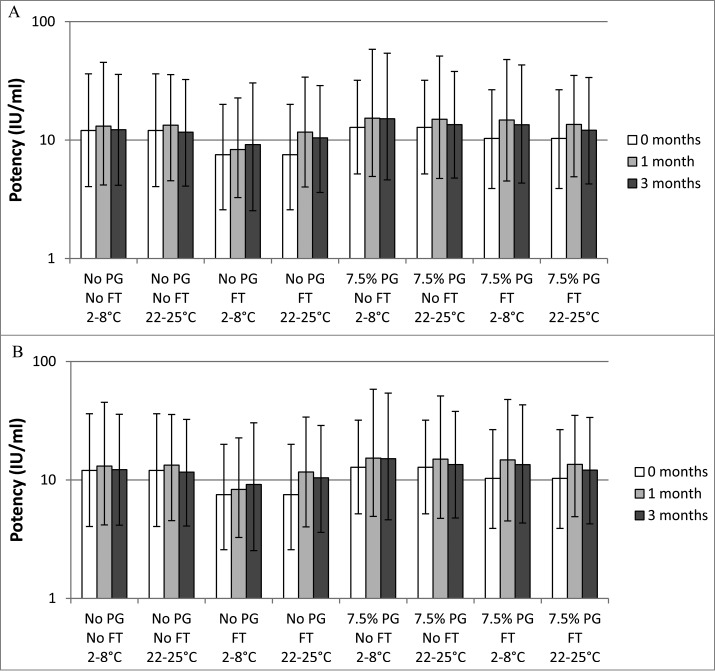
Freeze-thaw treatment of the DTwP and DTaP formulations without PG resulted in an immediate drop in potency of whole cell pertussis and acellular pertussis antigens to below the 8 IU/mL minimum requirement (Fig. 1A and B). PG (7.5% v/v) provided complete protection against freeze-thaw damage of whole cell pertussis (Fig. 1A). In the presence of PG, the potency of whole cell pertussis remained above the 8 IU/mL threshold (the lower limit of 95% confidence interval was above 2 IU) after 3 freeze-thaw treatments and throughout the 3-month stability test at both temperatures.
Figure 1.
In vivo potency of whole cell pertussis (Fig. 1A) and acellular pertussis (Fig. 1B) components of vaccines formulated with or without 7.5% (v/v) PG. The vaccines were exposed to freeze-thaw treatment (FT) or not (no FT) and stored at the indicated temperature for 0, 1, or 3 months before being used to immunize mice to test for their ability to protect against intracerebral challenge. IU: international units; FT: freeze-thaw; PG: propylene glycol. The error bars represent the upper and lower 95% confidence intervals. Potencies of acellular pertussis and whole cell pertussis vaccines were determined using the modified intracerebral challenge assay.9 NIH/OlaHsd mice were immunized intraperitoneally with 0.5 mL of serial dilutions of test vaccines or acellular pertussis reference vaccine. After 21 days, the mice were challenged by intracerebral injection of 100 to 1,000 LD50 of Bordetella pertussis organisms and observed daily for 14 d for paralysis and death. The number of surviving animals was recorded. Mice that died within 72 hours of the challenge were excluded from the analysis. The percentage of animals surviving at each dilution was used to calculate the potency compared with the international standard using the Reed-Muench equation and converted to potency IU per mL.
The results with acellular pertussis also showed that PG protected against freeze-thaw damage. The initial potency of acellular pertussis in the control vaccine was approximately 8 IU/mL. In the presence of PG, acellular pertussis retained its potency immediately after the freeze-thaw treatment and during the 3-month stability study. However, a sample with freeze-thaw treatment stored at 22°–25°C for 3 months appeared to have lower potency (Fig. 1B).
We conducted in vivo potency assays to investigate the protective effect of 7.5% (v/v) PG on diphtheria and tetanus toxoid potency in DTwP. Because DTaP was prepared using the same lots of diphtheria and tetanus toxoids used to prepare DTwP, we did not study the potency of diphtheria and tetanus toxoids in the DTaP vaccine.
Following freeze-thaw treatment, the potency of diphtheria toxoid in the PG formulation was equivalent to the untreated controls (Table 2). There was no further decrease in diphtheria potency in the freeze‑thawed vaccine over the 3-month storage period at 2°–8°C or 22°–25°C. All samples remained above the potency threshold of 60 IU/mL (the lower limit of 95% confidence interval was within 50%).
Table 2.
In vivo potency (IU/mL) of diphtheria and tetanus toxoid antigens in DTwP vaccines formulated with or without 7.5% (v/v) propylene glycol
| Diphtheria potency (95% CI) | Tetanus potency (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| No PG, no FT | PG, FT | No PG, no FT | PG, FT | |||||
| Time points (months) | 2°–8°C | 22°–25°C | 2°–8°C | 22°–25°C | 2°–8°C | 22°–25°C | 2°–8°C | 22°–25°C |
| 0 | 67.2 (54.5–82.8) | 67.2 (54.5–82.8) | 62.2 (50.7–76.0) | 62.2 (50.7–76.0) | 145.1 (100.1–211.2) | 145.1 (100.1–211.2) | 136.4 (72.7–202.5) | 136.4 (72.7–202.5) |
| 1 | 69.8 (53.7–90.6) | 65.9 (51.6–86.2) | 75.2 (66.8–104.8) | 63.5 (47.7–85.4) | 153.6 (108.3–213.7) | 136.1 (93.9–195.3) | 137.9 (96.7–195.4) | 123.8 (87.5–176.9) |
| 3 | 73.5 (58.8–92.0) | 59.8* (41.8–91.3) | 61.3 (49.2–76.0) | 64.1 (52.4–81.6) | 164.2 (110.6–255.1) | 114.3* (78.6–167.0) | 121.4 (81.6–183.1) | 114.7* (77.4–171.6) |
The vaccines were exposed to freeze-thaw treatment (FT) or not exposure (no FT) and stored at the indicated temperature for 0, 1, or 3 months before testing using in vivo potency assays; IU: international unit; PG: propylene glycol; FT: freeze-thaw.
Diphtheria toxin potency was determined using an in vitro toxin neutralization test. NIH/OlaHsd mice were immunized with 0.5 mL of serial dilutions of test vaccines or reference toxoid.9 Mice were bled 5 weeks after immunization and serial dilutions of heat-inactivated serum were incubated with diphtheria toxin before being added to Vero cell monolayers. After 6 d of incubation (37°C, 5% CO2), cell death was scored by pH change as indicated by a color change in the culture medium and visual inspection of the monolayers, and the potency of the diphtheria toxoid was calculated.
To assess tetanus toxin potency, NIH mice were immunized with serial dilutions of test vaccines or tetanus toxoid reference vaccine. After 4 weeks, the test groups were challenged subcutaneously with 50 LD50 (in 0.5 mL) tetanus toxin per mouse. The control group was challenged with 1 LD50 (in 0.5 mL) tetanus toxin per mouse. All groups were observed daily for mortality for 5 d The potency of the toxoid was calculated from the survival dose‑response curve.
For both the D and T potency assays, the percentage of animals surviving at each dilution was used to calculate the potency compared with the international standard using the Reed-Muench equation and converted to potency IU per mL.
*Potency of sample is below the minimum required potency.
The potencies of tetanus toxoid in both the test and control vaccines were comparable at all time points (Table 2). All vaccines had potencies above the 120 IU/mL threshold with the exception of the 3‑month time point at 22°–25°C, at which point both control and test vaccine had lost some potency.
Thus the in vivo potency results for diphtheria and tetanus toxoids were consistent with the particle size data, which showed that PG was able to prevent freeze-thaw induced agglomeration. Earlier studies have shown that the freeze-sensitivity of tetanus toxoid in DTwP vaccines varies between different manufacturers. In these studies, in vivo potency was generally unaffected by freezing to −10°C, but was reduced by 0–29% by freezing to −30°C.11
In summary, this study shows that propylene glycol prevents freeze-induced agglomeration of the aluminum adjuvants in DTwP and DTaP and preserves the potency of the pertussis antigens. The stability of each of the components was unaffected by the addition of propylene glycol, indicating that propylene glycol may offer freeze protection to a wide range of vaccines, including expensive new vaccine such as pentavalent (DTP‑HepB-Hib), pneumococcal conjugate vaccines, human papillomavirus vaccines, and many other vaccines
Acknowledgments
The authors would like to thank Julian Hickling, Amy Wales, Marjorie Murray, Janet Saulsbury, Clint Posey, and Patricia Logan for their assistance in the development of this article.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported with funding from the Bill & Melinda Gates Foundation.
References
- 1. Matthias DM, Robertson J, Garrison MM, Newland S, Nelson C. Freezing temperatures in the vaccine cold chain: a systematic literature review. Vaccine 2007; 25(20):3980-6; PMID:17382434; http://dx.doi.org/ 10.1016/j.vaccine.2007.02.052 [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization (WHO) Guidelines on The International Packaging and Shipping of Vaccines. Geneva: WHO; 2005. [Google Scholar]
- 3. Kartoglu U, Ozgüler NK, Wolfson LJ, Kurzatkowski W. Validation of the shake test for detecting freeze damage to adsorbed vaccines. Bull World Health Organ 2010; 88(8):624-31; PMID:20680128; http://dx.doi.org/ 10.2471/BLT.08.056879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Techathawat S, Varinsathien P, Rasdjarmrearnsook A, Tharmaphornpilas P. Exposure to heat and freezing in the vaccine cold chain in Thailand. Vaccine 2007; 25(7):1328-33; PMID:17157419; http://dx.doi.org/ 10.1016/j.vaccine.2006.09.092 [DOI] [PubMed] [Google Scholar]
- 5. Edstam JS, Dulmaa N, Tsendjav O, Dambasuren B, Densmaa B. Exposure of hepatitis B vaccine to freezing temperatures during transport to rural health centers in Mongolia. Prev Med 2004; 39(2):384-8; PMID:15226050; http://dx.doi.org/ 10.1016/j.ypmed.2004.01.029 [DOI] [PubMed] [Google Scholar]
- 6. Braun LJ, Tyagi A, Perkins S, Carpenter J, Sylvester D, Guy M, Kristensen D, Chen D. Development of a freeze-stable formulation for vaccines containing aluminum salt adjuvants. Vaccine 2009; 27(1):72-9; PMID:18973782; http://dx.doi.org/ 10.1016/j.vaccine.2008.10.027 [DOI] [PubMed] [Google Scholar]
- 7. Braun LJ, Jezek J, Peterson S, Tyagi A, Perkins S, Sylvester D, Guy M, Lal M, Priddy S, Pizak H, et al. . Characterization of a thermostable hepatitis B vaccine formulation. Vaccine 2009;27(34):4609-14; PMID:19523912; http://dx.doi.org/ 10.1016/j.vaccine.2009.05.069 [DOI] [PubMed] [Google Scholar]
- 8. Wang L, Lei D, Zhang S. Acellular pertussis vaccines in China. Vaccine. 2012; 30(50):7174-8; PMID:23084855; http://dx.doi.org/ 10.1016/j.vaccine.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 9. Chinese Pharmacopoeia Commission Pharmacopoeia of the People's Republic of China. Beijing: Chemical Industry Press; 2010. [Google Scholar]
- 10. Nony P, Girard P, Chabaud S, Hessel L, Thébault C, Boissel JP. Impact of osmolality on burning sensations during and immediately after intramuscular injection of 0.5 ml of vaccine suspensions in healthy adults. Vaccine 2001; 19(27):3645-51; PMID:11395198; http://dx.doi.org/ 10.1016/S0264-410X(01)00098-6 [DOI] [PubMed] [Google Scholar]
- 11. World Health Organization (WHO) The effects of freezing on the appearance, potency, and toxicity of adsorbed and unadsorbed DPT vaccines. Wkly Epidemiol Rec 1980; 55:385-92 [Google Scholar]