Abstract
Rotavirus (RV) is a major vaccine-preventable killer of young children worldwide. Two RV vaccines are globally commercially available and other vaccines are in different stages of development. Due to the absence of a suitable correlate of protection (CoP), all RV vaccine efficacy trials have had clinical endpoints. These trials represent an important challenge since RV vaccines have to be introduced in many different settings, placebo-controlled studies are unethical due to the availability of licensed vaccines, and comparator assessments for new vaccines with clinical endpoints are very large, complex, and expensive to conduct. A CoP as a surrogate endpoint would allow predictions of vaccine efficacy for new RV vaccines and enable a regulatory pathway, contributing to the more rapid development of new RV vaccines. The goal of this review is to summarize experiences from RV natural infection and vaccine studies to evaluate potential CoP for use as surrogate endpoints for assessment of new RV vaccines, and to explore challenges and opportunities in the field.
Keywords: correlates of protection, IgA, mucosal, rotavirus, vaccines
Abbreviations
- RV
rotavirus
- WHO
World Health Organization
- GAVI
Global Alliance for Vaccines and Immunisation
- CoP
correlate of protection
- VE
vaccine efficacy
- GE
gastroenteritis
- RV1
Rotarix®
- RV5
RotaTeq®
- HAI
haemagglutination inhibition
- MenC
Meningococcal serogroup C
- SBA
serum bactericidal assay
- rSAB
serum bactericidal assay using rabbit serum
- VEI
VE estimated with an immunological endpoint
- CO
cutoff
- RV-NA
RV specific neutralizing antibodies
- RV5-precursor
RV5 precursor reassortants
- RRV-TV
Rhesus RV-Tetravalent vaccine
- GMT
geometric mean titers
- EMA
European Medicines Agency
- RV-SIg
rotavirus secretory Ig
- ASC
antibody secreting cells
- mBc
memory B cells
- RV-T cells
rotavirus specific T cells
- SGE
severe gastroenteritis
Introduction
Rotavirus (RV) is a major vaccine-preventable killer of young children, contributing to a significant number of hospitalizations and acute, severe watery episodes every day in thousands of young children.1 Two RV vaccines are commercially available globally (Rotarix®, GlaxoSmithKlein Biologicals, Rixensart, Belgium; RotaTeq®, Merck Research, Pennsylvania, USA), have been WHO pre-qualified, and are being introduced in many countries worldwide. A new RV vaccine, RotaVac™ (Bharat Biotech International Ltd, Hyderabad, India) has recently been licensed in India based on good safety and efficacy data2 although it is not yet WHO pre-qualified for the GAVI market.
Due to the absence of a suitable correlate of protection (CoP), all RV vaccine efficacy (VE) trials have had a clinical endpoint (pathogen-specific morbidity and/or mortality). These trials represent an important challenge since RV vaccines have to be introduced in many different settings, placebo-controlled studies are unethical, and comparator assessments for new vaccines with clinical endpoints are very large, complex, and expensive to conduct. The consideration of a CoP as a surrogate endpoint would allow predictions of VE for new live, oral RV vaccines and enable a regulatory pathway, contributing to the more rapid development of new generation of RV vaccines, and support for guiding vaccination policies and regulatory decisions.
Natural RV infection does not generate sterilizing immunity, thus, reasonable goals of vaccination are to decrease or eliminate severe disease in children, but not to prevent infection. For this reason, the clinical endpoint for RV vaccines trials has been prevention of gastroenteritis (GE) and mainly severe RV disease. Other important endpoints include prevention of infection by specific strains of RV, hospitalization, and death due to RV.
The goal of this review is to summarize experiences from natural infection and vaccine studies to evaluate potential immune markers for use as surrogate endpoints for clinical assessment of new RV vaccines, and to explore challenges and opportunities in the field. Several reviews3-6 have summarized multiple aspects of RV immunity and potential CoP for RV vaccines. Here, we will only concentrate on the issues most directly related to the identification of CoP for RV vaccines, and, in particular, those related to the Rotarix® (RV1) and RotaTeq® (RV5), the commercially available RV vaccines used worldwide.
Definitions and Methods for Evaluating CoP
Definitions
Both definitions of general “clinical trial endpoints” and of CoP seem important in the context of this discussion:
Clinical trial endpoints
In clinical trials, the clinical endpoint is described as: “a characteristic or variable that reflects how a patient feels or functions, or how long a patient survives”,7 which serves as the target outcome of the study. In contrast, a “Surrogate Endpoint” is: “a biomarker intended to substitute for a clinical endpoint”.7 The latter “is expected to predict clinical benefit (or harm, or lack of benefit or harm) based on epidemiologic, therapeutic, pathophysiologic, or other scientific evidence”.7 Fleming et al.8 proposed the following hierarchy for clinical trial endpoints:
Level 1: a true clinical-efficacy measure;
Level 2: a validated surrogate endpoint;
Level 3: a non-validated surrogate endpoint, yet one established to be “reasonably likely to predict clinical benefit;"
Level 4: a correlate that is a measure of biological activity but that has not been established to be at a higher level.
Correlates of protection
The terminology in the literature describing the substitution of biological parameters for clinical endpoints in VE trials is heterogeneous.9 Importantly, it is necessary to clarify the level of empirical support existing for a CoP.10 Recently, Plotkin and Gilbert proposed the following new terminology, that adjusts very well to RV vaccines11:
CoP: an immune marker statistically correlated with VE (equivalently predictive of VE) that may or may not be a mechanistic causal agent of protection. In other words, individuals having better results for the biological marker are inclined to have better results for the clinical efficacy endpoint.8
Mechanistic CoP: a CoP mechanistic and causally responsible for the observed protection.
Non-mechanistic CoP: a CoP that is not a mechanistic causal agent of protection.
Absolute CoP: A quantity of a specific immune response to a vaccine that always provides near 100% protection.12
Relative CoP: A quantity of a specific immune response to a vaccine that usually (but not always) provides protection.12
For a Level 2 endpoint for vaccine trials (ie. a validated CoP), it is ideal to use a mechanistic CoP, which guarantees that any change affecting the biomarker will, in fact, affect the clinical endpoint. When using a non-mechanistic CoP this direct relationship may or may not exist.8 When a mechanistic CoP is used, any improvement in the vaccine formulation that is accompanied by an improvement in the mechanistic CoP will guarantee an improvement of the clinical endpoint. For these reasons, the efforts should concentrate in the identification of a mechanistic CoP.
However, from the immunological point of view, we agree with Follman13 when he states: “Strictly speaking, one cannot know whether the measured immune responses, or other unmeasured vaccine-induced changes, are actually responsible for an efficacious vaccine.” Chan et al.14 highlight a practical consequence for this fact: “While the concept of a CoP usually refers to establishing a protective level of antibody titer, identifying a clear-cut value is often impossible because VE is not related solely to the antibody titer”.
Methods for investigating vaccine CoP
The methods for investigating vaccine CoP depend on the type of pathogen the vaccine is intended to protect from.10 RV belongs to the group of pathogens for which individuals can undergo multiple re-infections, and an immunological parameter may vary in both unvaccinated and vaccinated subjects, so that it can be evaluated as a CoP analyzing both types of participants in a vaccine trial.10 Consequently, we will concentrate in the statistical models that apply to this type of pathogens.
For VE trials the relevant statistical question is how to model and validate the relationship between immune assay values (very frequently antibody levels) and protection from disease.15 Discussions in the literature on this subject have been frequently based on postulates for evaluating surrogate trial endpoints proposed in 1989 by Prentice.8,10,16,17 Prentice's first condition is that a valid surrogate endpoint must be correlated with the true clinical endpoint.17 Most candidates for CoP fulfill this condition.10 However, even at this first level of surrogacy there are important challenges: management of potential measurement errors of the putative CoP, time variations of the CoP, and development of an efficient sampling design that adequately incorporates participant covariate information.18 Prentice's second condition is very restrictive: the surrogate endpoint needs to fully capture the treatment's “net effect” on the true clinical endpoint. This means that if the value of the CoP for an individual is identified, knowledge of treatment provides no additional prognostic information.16 Thus, only true mechanistic CoP fulfills this condition. For this reason, Freedman et al.19 introduced the notion of ‘proportion explained’, which is the proportion of the treatment effect mediated by the surrogate. However, some authors are skeptical about this type of analysis.9,20
Statistical analyses for individual VE trials
In numerous vaccine studies, a threshold level of an immune assay above which subjects do not develop disease have been identified.15 Individuals who achieve the threshold after vaccination are considered protected. Associations between protection and a minimal threshold antibody titer are usually analyzed using the Chi-square test (x2) or Fisher's exact test. Another approach is to use a minimal protective threshold antibody titer as a categorical variable in a logistic regression model. In studies where both unvaccinated and vaccinated subjects can be evaluated, VE calculated with a clinical endpoint is compared to VE estimated with an immunological endpoint (VEI).21 For the clinical endpoint: VE = 1 – (ARV/ARU), where ARV is the attack rate in vaccinated individuals and ARU is the attack rate in unvaccinated individuals. Similarly, VEI = 1− (Iv < CO)/(Iu < CO), where (Iv < CO) and (Iu < CO) indicate the probabilities that vaccinated and unvaccinated individuals have less than the cutoff (CO) level of the immune parameter (I), respectively. In other words, the relative risk of disease equals the relative risk of having antibodies less than the CO. In this analysis it is important that at the different titers of I, the probability of infection be the same in vaccine and placebo recipients, indicating that I functions equally in both groups.
Some limitations of the threshold model, as presented by A.J. Dunning,15 are: 1) The relationship between assay values and the occurrence of disease below the threshold level is frequently not specified. In this case, the rate of disease among individuals with low immune assay values may be more strongly associated with the risk of exposure and disease prevalence rates, variables not taken into account in the threshold model. Thus, the model does not provide a suitable method for fully predicting VE. 2) Subjects with assay values above a chosen threshold will occasionally develop disease. Taking into account these issues, models for calculating titer-specific rates of disease22 or that complete the unspecified part of the threshold model15 have been developed. Logistic regression is also used to quantify the relationship between different immune assay values and the occurrence of disease.10,23-25 Such analysis for example would provide for RV vaccines the likelihood of not having a RV associated-GE with each 1 log increase in antibody titer.
Meta-analytic models for combining information from multiple studies
Ideally, a CoP should be equally useful for multiple vaccines and/or different settings. Various meta-analytical models are used to validate CoP for this purpose.9,26,27 Of note, it is generally recommended that before performing the meta-analysis the candidate CoP be recognized as such at the individual trial level.10
Experiences with Other Vaccines
There are existing models where surrogate CoP have been utilized and which are informative to this discussion.
Influenza virus vaccines
Support for the use of serum haemagglutination inhibition (HAI) titers as a surrogate endpoint for influenza VE has been constructed over time. In the first efficacy trials of an inactivated influenza vaccine, the authors found that higher HAI titers were associated with protection in both vaccinated and unvaccinated individuals.28 Subsequent studies29,30 also demonstrated that HAI antibody was a major determinant of protection, and in further studies, HAI titers of 40–60 prior to infection were clearly associated with protection from infection.31,32 Of note, these reports were prior to the publication of Prentice referenced above.17 However, Qin et al.10,18 reanalyzed the data from the first influenza vaccine trial28 for observed and predicted strain-specific infection incidences from logistic regression fits of the log2 titers of strain specific antibodies. They found that, measurement of titers of antibody to Weiss strain A, but not to PR8 strain A, satisfied the Prentice criterion as a perfect surrogate endpoint: it fully captures the treatment's “net effect” on the true clinical endpoint.10
More recently, the current policy, requiring new influenza vaccines to induce serum HAI titres ≥40 in the majority of vaccinees, was supported by a meta-analysis of clinical data.33 However, higher levels of HAI titers apply for children34 and experts have highlighted several unsolved issues in the field.35,36 Also, extrapolation to pandemic influenza of the CoP for seasonal influenza is not recommended, because it is thought that protection against the former type of virus reflects the sum of various immune responses, including antibody and cell-mediated responses.37
Meningococcal vaccines
Meningococcal serogroup C (MenC) conjugate vaccines are highly immunogenic, and generate functional antibodies as measured by the serum bactericidal assay (SBA).38 Studies during the 1960s showed that adults with naturally acquired SBA titers ≥1:4 (using human serum as exogenous complement source) were protected from MenC disease.39 The United Kingdom Medicines Control Agency therefore accepted estimations of MenC VE based on immunogenicity using the SBA.38 Later on, the SBA was improved using rabbit serum (rSAB) as an exogenous complement source.38 rSBA titres of <1:8 were predictive of susceptibility to invasive meningococcal disease, and titres of ≥1:128 were predictive of short-term protection. Besides, it was proposed that for children with rSBA titers between 8 and 64, mostly toddlers, additional serological criteria would be required for the presumption of protection (see below).38 Since the majority of toddlers met the additional criteria, Andrews et al.21 used postlicensure surveillance data to identify the rSBA threshold level to predict protection at different ages. The authors compared the results of VE calculated with clinical endpoint vs VEI, as described above. They found that rSBA titres of ≥1:8 were predictive of short-term protection for toddlers.21
Pneumococcal conjugate vaccine
WHO sponsored a consultation in 2002 to provide guidance for evaluation of new pneumococcal conjugate vaccines based on serological criteria.40 At the time, there was no clear agreement on the concentration of antibody that could be used to predict VE. This situation is very similar to the present one in the RV vaccines field, where a CoP is needed to help guide new RV vaccines development and clinical evaluation. The first step was to choose a surrogate endpoint for VE trials, and participants agreed that a single primary immunological endpoint was sufficient for registration of new vaccines. IgG antibody concentrations, as measured by ELISA 4 weeks after a 3-dose priming series, appeared to be the best parameter to use as the primary criteria, because: “1) IgG is the desired type of immune response; 2) the methodology for measuring this parameter was validated in infants; 3) a bridge to efficacy data had been established and, 4) a cross-laboratory standardization process had been completed”.40 The IgG quantified by ELISA is a surrogate measurement for the likely protective activity (bactericidal or opsonic antibody), hence, it is a non-mechanistic CoP.
Next, they proposed that a single antibody threshold level would be determined through an analysis pooling data from the efficacy trials, with invasive disease endpoints, that were available. In addition, it was proposed that the percentage of responders should be used as the criteria to determine non-inferiority of a new vaccine. After the analysis of 3 double-blind controlled VE trials, a concentration of IgG anti-capsular polysaccharide antibodies measured by ELISA ≥ 0.35 μg/ml was adopted as the protective threshold for all pneumococcal serotypes.41 The analysis was based on the notion of VEI described above, but, since VE calculated with a clinical endpoint was known for all trials, the protective antibody threshold was directly determined from the reverse cumulative distribution curves42 of the antibody concentrations of the vaccinated group and the control group.41
Analysis of CoP After Natural RV Infection or Vaccination
Correlates of protection after RV natural and experimental infection
Aside from studies in animal models, much has been learned of the mechanisms of protection against RV in humans with experiments in which adult volunteers are experimentally challenged with RV, or by studying children with natural infections. Evidence from these studies are briefly reviewed in what follows:
Serotype specific neutralizing antibodies
Classically, RV specific neutralizing antibodies (RV-NA) are directed against the outer viral proteins, VP4 and VP7, which are involved in viral attachment and entry to cells. Two types of RV-NA have been described: homotypic (blocking only one RV serotype) or heterotypic (blocking 2 or more RV serotypes).6,43,44 Studies evaluating associations between serum RV-serotype specific antibodies and protection are summarized in Table 1.
Table 1.
Studies evaluating associations between serum RV-serotype specific antibodies and protection
| Setting | n | Clinical endpoint | Type of assay for NA | Titer | Stat Test | P= | Ref |
|---|---|---|---|---|---|---|---|
| Adults challenged with human RV | 16–18 | GE | NA against homotypic RV | ≥1:100 | FETa | 0.044 | 47 |
| EBAb VP7 epitopes Homotypic (G1) Heterotypic (G3) | ≥1:20 | FET | 0.0007, 0.02 | 49 | |||
| Infection (and GE) | ICAc IgG to homotypic VP7 (G1) | 1:6,607 d | LRAe | <0.008 | 50 | ||
| ICA IgG to homotypic VP4 P1A[8] | 1:3,716 d | LRA | 0.009 | 50 | |||
| Adults challenged with human RV | 38 | GE and infection | NA against homotypic and heterotypic RV | ND f | LRA | NS g | 46 |
| Orphanage | 44 | GE | NA against homotypic RV | ≥1:128 | None | 51 | |
| Daycare center | 60 | Infection | EBA homotypic VP7 epitopes | ≥ 44% blocking level | X2 | <0.001 | 52 |
| Case-control study in Bangladesh | ≥ 79 | GE | NA against heterotypic RV | ND | LRA | <0.05 | 53 |
a FET: Fischer exact test.
b EBA: Epitope blocking assay that measures response to specific neutralizing epitopes.
c ICA: immunocytochemical assay.
d Titer that predicted 75% probability of resistance to RV infection.
e LRA: Logistic regression analysis.
f ND: not determined.
g NS: Non significant.
RV-NA detected in intestine: It is generally accepted that NA against the infecting strain present in the intestine provides protection.6,43–45 Thus, if measurement of intestinal RV-NA were used as an endpoint in VE trials, it would probably be considered a true mechanistic CoP. However, in studies with adults experimentally challenged with homologous human RV, the relationship of pre-existing NA in intestinal fluid with protection was observed in one study (using a stepwise logistic regression, p = 0.01)46 but not in another (using a 2-tailed Fisher exact test, p = 0.49, cut off 1:100).47 Most authors agree that this lack of definitive experimental evidence is mainly due to unsolved technical issues of the assay. The evaluation of NA in intestinal fluid is not practical for clinical studies,48 and measurement of stool RV-NA also presents practical and technical problems (see below).
RV-NA detected in serum: A significant relationship between pre-existing serum homotypic RV-NA and protection against RV GE was found in adults experimentally challenged with homologous human RV47 (Table 1). Neutralizing VP7 epitopes that correlated with protection were identified in this study.49 Furthermore, it was recently shown that titers of IgG antibody to homotypic VP7 and VP4 (that may reflect NA) were highly correlated with the probability of resistance to RV infection (mostly symptomatic infection).50 In contrast, the association between serum homotypic or heterotypic NA was not detected in another study of adults experimentally infected with a different RV.46 A relationship between serum RV-NA and protection against natural infection has been observed in children attending an orphanage51 or a daycare center,52 and in a case-control study in rural Bangladesh.53 Nonetheless, this relationship has not been observed in another community study.54
Total non-neutralizing RV-specific antibodies (IgA, IgG)
Intestinal and stool RV-IgA: Since measurement of intestinal RV-NA is impractical, other types of samples and antibodies have been examined to detect an immune response after natural infection.48 An association between duodenal and fecal RV-IgA titers after natural RV infection in children was found.48 Stool RV-IgA had a sensitivity of 73% and 92% predictive accuracy for duodenal RV-IgA levels, 4 months after RV infection.48 Another study showed that RV-IgA copro-conversion had a sensitivity of 92% and a predictive accuracy of 92%, for estimating RV reinfections,55 and was proposed as a potential CoP after vaccination.
However, few published studies have assessed stool RV-IgA as a CoP (Table 2). In adults experimentally infected with RV, no correlation was observed between stool RV-IgA (or jejunal RV-IgA) and protection against RV GE or infection.46 In contrast, in a daycare center study, higher stool RV-IgA antibody titers were associated with protection against infection (≥1:80) and illness (≥1:20).56 Nonetheless, there were significant numbers of children with elevated pre-infection stool RV-IgA titers that became infected. In an Australian cohort study, frequent RV infection of children appears to stimulate production of sustained levels of fecal IgA (plateau levels) and lower ratio of symptomatic to total number of RV reinfections; however, the fecal RV-IgA was not long lasting in a subset of children, with a duration of a few weeks.57
Table 2.
Studies evaluating associations between stool RV-IgA and protection
| Setting | n | Clinical endpoints | Titer | Statistics | P= | Ref |
|---|---|---|---|---|---|---|
| Adults challenged with human RV | 38 | Infection and GE | ND | Stepwise logistic regression | NS a | 46 |
| Daycare center | 100 | Infection | ≥1:80 | X2b | 0.026 | 56 |
| GE | ≥1:20 | X2b | 0.015 | 56 |
a NS: Non significant.
b Chi Squared.
Serum RV-IgA: Serum RV-IgA was elevated in 93% of children and showed 100% sensitivity and 79% predictive accuracy for duodenal RV-IgA levels 4 months after natural RV infection.48 For children attending a daycare center in Texas, total serum RV-IgA titers of >1:200 were associated with protection against infection52 (Table 3). Probably the best available evidence for serum RV-IgA as CoP after natural infection comes from a study with a cohort of 200 Mexican children that were followed from birth until 2 y of age. Children with a serum RV-IgA titer >1:800 had a lower risk of RV infection (adjusted relative risk [aRR], 0.21; P < 0.001) and diarrhea (aRR, 0.16; P = 0.01) and were completely protected against moderate-to-severe RV GE.58,59
Table 3.
Studies in which serum RV-IgA and RV-IgG have been associated with protection
| Setting | n | Clinical endpoint | IgA Titer | IgG Titer | Statistics | P= | Ref |
|---|---|---|---|---|---|---|---|
| Adults challenged with human RV | 38 | Infection | NDa | Stepwise logistic regression | 0.005 | 46 | |
| Case-control study Bangladesh | 179 each | GEb | 100–199 U/ml | X2 ORc 0.25 | <0.05 | 60 | |
| Daycare centerd | 63 | Infection | >1:200 | X2 with Yates's correction | 0.001 | 52 | |
| >1:800 | X2 with Yates's correction | <0.001 | 52 | ||||
| Mexican cohort | 200 | Infection | > 1:800 | GEEe aRR, 0.21 | <0.001 | 58 | |
| GE | > 1:800 | GEE aRR, 0.16 | 0.01 | 58 | |||
| Infection | >1:6400 | GEE aRR, 0.51 | <0.001 | 58 |
a ND: not determined.
b Analyses were performed in children ≥8 months of age with negligible titers of circulating maternal antibodies.
c Odds ratio.
d Analysis adjusted for age: Mantel-Haenszel X2 P = 0.07 for IgA; X2 P = 0.04 for IgG.
e Generalized estimating equation (GEE) models reporting adjusted relative risks (aRR).
Serum RV-IgG: Serum RV-IgG was elevated in 100% of children and showed 100% sensitivity and 79% predictive accuracy in relation to elevated duodenal RV-IgA levels 4 months after natural RV infection.48 In adults experimentally infected with RV serum RV-IgG was associated with protection from infection46 (Table 3). Mexican children with an IgG titer of >1:6400 were protected against RV infection (aRR, 0.51; P < 0.001), but not against RV GE.58,59 For children attending daycare centers in Texas a RV-IgG titer of >1:800 was associated with protection against both infection and illness.52 Finally, in the case-control study in rural Bangladesh, an association between RV-IgG titers of 100–199 U/ml and protection against GE was also observed.60
In conclusion, total serum RV IgA, serum homotypic and heterotypic NA, and maybe to a lesser degree serum RV-IgG, have been correlated with protection induced by natural infection in children.4
Correlates of protection after vaccination
Technical aspects of the immunological assays used
The measurement of IgA responses to the precursor strain of RV1 (89-12), and to RV5 and the RV5 precursor reassortants (RV5-precursor) vaccines have been performed in the laboratory of Drs. Ward and McNeal in Cincinnati (Table 4). The responses against RV1 were performed by GSK using a protocol derived from the one used for 89-12 (Table 4). Thus, these assays are very similar and the results should in principle be comparable. For a typical experiment, ELISA plates are sequentially treated with: 1) rabbit anti-RV IgG; 2) lysate of virus-infected cells (WC3,61 89-12,62 and presumably the RIX4414 virus lysates for the RV5, 89-12, and RV1 vaccines, respectively) or the lysate of mock-infected cells used as a negative control; 3) a human serum pool (used as a RV-IgA standard) or test serum samples (starting with a dilution of 1:20) are added to both wells with or without virus; 4) Biotinylated rabbit anti-human IgA and finally Peroxidase Conjugated Avidin/Biotin and a colorimetric developing reagent.
Table 4.
RV-IgA seroconversion/seropositivity/seroresponse rates in selected RV vaccine trials
| Vaccine/ Ref | Site | OPV | IgA Seroconversion (C) Seroresponse (R) Seropositivity (P) in vaccinees % (95% CI) | Cut Off | Time after last vaccine dose | Baseline and rise criteria |
|---|---|---|---|---|---|---|
| 89-1262 | USA | NRa | 95 (C) | >4U | 20–27 days | ≥4 Fold rise in 20 PUVb |
| 89-1263 | USA | No | 91.6 (P) | ≥20 U | 21–35 days | ≥20 U in 107 Vc (no PId sample) |
| RV1102 | Latin America | Yes | 61.4 (53.7 to 68.6) (P) | ≥20 U | 1 to 2 months | ≥20 U in 300 V (no PI sample) |
| RV1¶103 | Europe | No | 86.5 (83.9 to 88.8) (C) | ≥20 U | 1 to 2 months | ≥20 U in 787 PUV |
| RV1¶104,105 | Latin America | No | 76.8 (72.4 to 80.9) (C) | ≥20 U | 1 to 2 months | ≥20 U in 393 PUV |
| RV1¶64 | Africa | Yes | 53.8 (43.8 to 63.5) (C) | ≥20U | 2 months | ≥20 U in 106 PUV (2 vaccine doses) |
| 65.2 (62.4 to 67.9) (P) | ≥20 U | 2 months | ≥20 U in 1160 V (no PI sample, 2 vaccine doses) | |||
| PRV561 | USA | NR | 94.6 (C) | ≥10 U | 2–4 weeks | 3 Fold rise in 37 PUV |
| RV572 | Finland, USA | No | 95.2 (91.2 to 97.8) (C) | NR | 14 days | 3 Fold rise in 189 V (not established if PUV) |
| RV567,71 | Africa | Yes | 78.3 (71.7 to 84.0) (R) | NR | 14–21 days | 3 Fold rise in 148V |
| RV5101 | Asia | Yes | 87.8 (80.9 to 92.9) (R) | NR | 9- 33 days | 3 Fold rise in 115V |
aNR: not reported.
bPUV: Previously uninfected vaccinees as determined by the presence of RV-IgA.
cV: vaccinees.
dPI: preimmune.
¶GlaxoSmithKline, Clinical study registers available at: http://www.gsk-clinicalstudyregister.com/ Accessed 9 January 2012.
The number of RV-IgA units in a test sample is calculated from the standard curve by interpolation. COs and criteria for interpretation of the test have varied between the different studies (Table 4). In the early 89-12 studies: “20 units/mL IgA was chosen as the cut-off point because it is well above the assay limit of detection (5 times) and it has been previously used as evidence of a natural RV infection.”.63 Although in the early RV5-precursor studies, 10 units/ml was chosen as a CO, this seems to have been abandoned in the later studies in which increments between pre-immune samples and samples taken after the last vaccine dose are reported as “seroresponse” rates and not seroconversion rates (Table 4).
A seroresponse is defined by a ≥3 fold rise in RV-IgA units between the pre-immune and the sample taken after the last vaccine dose (Table 4). For the RV1 studies, in which pre-immune samples were not available, results are reported as seropositivity rates and, when pre-immune samples are available, as seroconversion rates (Table 4). Seroconversion is defined as the appearance of antibodies (≥20 units) in the sera of subjects seronegative (<20 units) before vaccination. As expected, in trials in which both seropositivity and seroconversion rates have been evaluated the latter are higher than the former64 (RV1 trial in Africa; Table 4). Finally, another variable that has differed between studies is the time after the last vaccine dose at which the serum sample is taken to measure RV-IgA. This has varied from 4–8 weeks in the RV1 studies compared to 2–4 weeks in the RV5 studies (Table 4).
The measurement of serum RV-NA for the 89-12 and RV5 vaccines were performed in Cincinnati by an antigen-reduction assay.65 Seroconversion rates in RV-NA for both 89-1266 and RV567 have been, in general, lower than for RV-IgA and the protection induced by the vaccines. Similar to RV-IgA, interpretation criteria between the 89-12 and RV5 studies also exist for RV-NA: for the 89-12 studies seroconversion in RV-NA was defined by a ≥4 fold increase, while in the RV5 studies a ≥3 fold increase has been the standard.67 Pre-existing serum RV-NA in the infants, presumably of maternal origin, may hinder the measurement of those induced by the vaccine: In some cases, serum RV-NA is higher in the pre-immune sample than in samples taken after the last dose of the vaccine, probably reflecting maternal antibody.67
Assessment of serum RV-NA after vaccination
Rhesus RV-Tetravalent vaccine (RRV-TV): In a clinical trial with low doses (4 × 105 pfu) of RRV-TV vaccine, logistic regression analyses were performed to identify associations between serotype-specific NA titers and protection against RV GE.68 A significant (P = 0.03) association was found between post–dose 3 anti-Wa (serotype G1) titers and protection against serotype G1 illnesses. There was also an association between anti-RRV NA (P < 0.001) and protection against serotype G3 illnesses. However, such association was not observed with antibodies against another non-vaccine G3 RV strain. Moreover, for the antibodies that correlated with protection, no specific titers could be associated with protection against serotype G1 or G3 illnesses.68
RV5 and its precursors: In an early trial of the WC3 vaccine, vaccinees that developed WC3 NA titers of ≥1:160 (P < 0.03, odds ratio 0.24, CI 0.06–0.85) or ≥1:200 (P < 0.004) developed less RV GE (Fisher's exact test or x2 analysis).69 However, overall effectiveness of the WC3 vaccine was not demonstrated in this trial. In contrast, in clinical trials in which WC3 was protective, an immune correlate of protection was not identified.70 Moreover, correlations with protection were also not found in early studies with various WC3/HRV reassortants.70 In at least 3 very large studies with RV5 in different settings the rates of serum RV-NA and protection have been determined simultaneously; in most cases protection against disease has exceeded the seroresponse rate.67,71-73 No statistical analysis of serum RV-NA as a CoP in these studies has been presented.
RV1 and its precursors: Only 35% of children who received a highly protective dose of the 89-12 human attenuated RV vaccine developed serum RV-NA to the vaccine virus, suggesting that these antibodies are not good CoP for this vaccine.62 Moreover, the poor capacity of children to develop serum RV-NA was associated with the presence of maternal antibodies, and age at immunization. Children vaccinated at 4 or 6 months of age showed higher RV-NA seroconversion rates (75–79%), but are, nonetheless, lower than RV-IgA seroconversion rates and the protective efficacy of the vaccine.66
In conclusion, evidence for the usefulness of measurement of serum RV-NA as a surrogate endpoint for RV VE, in an important number of trials, is weak. The masking of vaccine induced serum RV-NA by maternal antibodies may in part explain this finding.
Assessment of total RV-IgA after vaccination
Stool RV-IgA: Stool RV-IgA responses were examined after vaccination with the RV5-precursor, but statistical analysis for this marker as a CoP has not been presented.45,74 Moreover, there seems to be an agreement that measurement of stool RV-IgA is technically difficult, and the presence of maternal milk RV-IgA may mask the detection of vaccine induced RV-IgA.75 It seems doubtful that stool RV antibodies can be used as surrogate endpoint for RV vaccines.
Serum RV-IgA: When RV-IgA and RV-IgG have been directly compared to evaluate vaccine immunogenicity, RV-IgA seems to have been the most sensitive,76 and RV-IgA has been routinely measured during RV vaccine trials. These responses have been successfully used as markers for vaccine “take." However, few studies have tried to associate RV-IgA with protection after vaccination (Table 5). These studies, reviews, and recent meta-analysis are considered below.
RRV-TV vaccine: Seroconversions for RV-IgA in RRV-TV vaccinees were significantly associated with protection from infection, but not GE (Table 5), in a trial with low doses of vaccine.68 Significant associations with protection against GE were found for post–dose 3 titers of RV-IgA in vaccinees, however, no specific titer could be identified as a marker of protection for RRV-TV vaccine. No correlate of immunity against RV infection or disease was identifiable based on seroconversion to any of the antibodies measured in children vaccinated with low dose of RRV-TV (4 × 104 pfu)77 or against disease with titers detected at the beginning of the GE (4 × 105 pfu) 78 using Fisher exact tests.
RV5 and its precursors: Serum RV-IgA has been measured in reported RV5 trials, but its association with protection has not been formally addressed. In these studies the rates of RV-IgA seroresponse exceed, in several cases, the rates of protection against severe RV GE3,4 (see Fig. 1 and the discussion below).
RV1 and its precursors: An association between serum RV-IgA and protection in children vaccinated with low doses of RV1 was suggested in a study in Finland79 (Table 5). An early analysis of several RV1 studies concluded: “Serum RV IgA responses in Rotarix® vaccinees have directly reflected the efficacies of this vaccine. Vaccinees that did not develop RV IgA (non-responders) were about 10 times more likely to experience a subsequent RV illness during the first season than responders.”45 A more recent analysis utilized 2 models to evaluate the role of serum RV IgA.25 In the first model, individual subject data from a large, Phase III, RV1 efficacy study in Malawi (involving 1,773 infants) and South Africa (involving 3,166 infants) was used for logistic regression analysis to assess the relationship between protection against RV and post-vaccination anti-RV IgA antibody. A RV-IgA titer equal or higher than 20 U/mL was associated with protection against RV GE. Nonetheless, vaccinees without RV-IgA response (less than 20 U/ml) had significantly less severe RV GE (or any RV GE) than placebo recipients, suggesting that the vaccine protects through mechanisms that are not fully captured by the RV-IgA assay. In the second model, a meta-analysis of different populations from 8 RV1 efficacy studies in Europe, Asia and South America using linear regression found a correlation between RV-IgA VEI rates and VE. The authors concluded that while IgA is a potentially valuable epidemiological tool at the population level, it cannot be used to predict individual protection.
Factors affecting both RV5 and RV1 vaccines: Differences in RV-IgA responses can vary not only because of technical and interpretation criteria of the ELISA, but also because of characteristics of the settings where the vaccine trials take place (Table 4). As for other oral vaccines, RV vaccines have consistently been less efficient in low-income countries. Several factors can modify the accuracy of a CoP in these countries: a) the challenge dose, which can be higher in some low-income countries, could influence the quality and quantity of a CoP.12 b) Presence of maternal antibodies in serum of young children also modulates the immune response to the vaccines.80 Analyses of covariance indicated that higher pre-vaccination serum RV-NA titers of maternal origin negatively affected post-vaccination titers of RV-NA titers to the RRV-TV vaccine.68 Also, a consistent inverse relationship between transplacental RV-NA to RV1 and RV-IgA titers following immunization has been found in countries representing all socioeconomic levels.45 c) In low-income countries and some middle-income countries RV vaccines have been administrated simultaneously with OPV (Table 4). In these studies, consistently lower levels of serum RV-IgA have been observed.81 These results suggest that OPV impacts RV-IgA as CoP for RV1. Patel et al.24 conducted a systematic review of the immunogenicity and VE studies of RV5 and RV1 and stratified the data into low-, medium- and high-mortality countries for children under 5 (based on WHO standards). RV-IgA titers correlated inveresly with under-5 mortality for both RV1 and RV5. This result suggests that the titers (“units”) of RV-IgA calculated for both types of vaccine studies are comparable and higher in countries where the vaccines work best. However, the relationship between RV-IgA and protection seems to be somewhat different for the 2 vaccines (Fig. 1).
Table 5.
Studies evaluating associations between serum RV-IgA and protection in vaccinated children
| Vaccine | n | Clinical endpoint | IgA | Statistics | P= | Ref |
|---|---|---|---|---|---|---|
| RRV-TV 4 × 105 pfu | 193 vac 205 plac | Infection | Seroconversion (4-fold- increase) | Fisher exact test | 0.01 | 68 |
| GE | Seroconversion (4-fold increase) | Fisher exact test | NSa 0.06 | 68 | ||
| GE | Post-dose 3 titers | Fisher exact test? | 0.01 | 68 | ||
| RV1 104.7ffu | 405 | GE | Seroconversionb | Fisher exact testc | 0.01 | 79 |
a NS: Nonsignificant.
b Percentages of infants with RV IgA antibody concentration of ≥20 units/ml in infants initially seronegative for RV IgA antibody before vaccination.
c Fisher exact test calculated by us with results from Table 3 from the original paper.
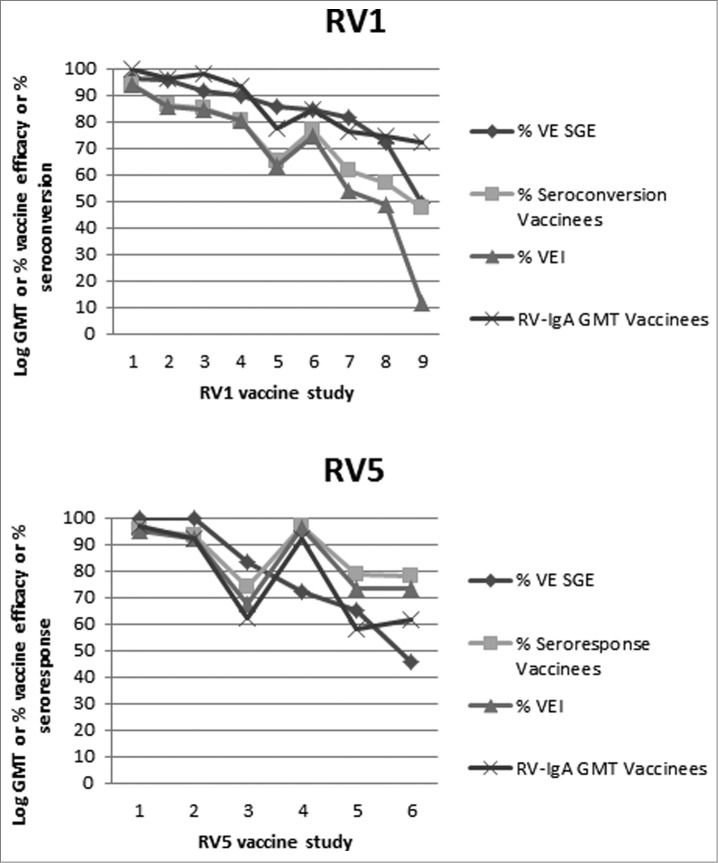
Figure 1.
Selected RV1 (top panel) and RV5 (bottom panel) trials are presented from left to right in order of decreasing VE against severe GE (SGE). For each trial the VE against SGE, seroconversion rate (RV1 trials) or seroresponse rate (RV5 trials), VEI and RV-IgA log GMT (the latter adjusted with a constant for scaling) are plotted. VEI was calculated based on seroconversion rates for RV1 and seroresponse rates for RV5 of vaccinees and placebo recipients. For RV1 the data was obtained from the GSK, Clinical study registers available at: http://www.gsk-clinicalstudyregister.com/ Accessed January 9 2012. The corresponding location and GSK Trial numbers are: 1: Asia 028–030, 2: Europe 036, 3: Japan 056, 4: Finland 004, 5: Latin America 006, 6: Latin America 023, 7: Latin America 024, 8: 037 in South Africa, 9: 037 in Malawi. For RV5 the trial references are: 1: Finland and US,98,99 2: Finland,99,100 3: Finland and US,72 4: Kenya,67,71 5: Vietnam,101 Ghana,67,71 6: Bangladesh.101
For RV1 the VEI and seroconversion rates are in general lower than the VE against severe RVGE. In contrast, for RV5 the VEI and seroresponse rates are for some trials higher and for others lower than the VE against severe RVGE. For RV1 the RV-IgA geometric mean titers (GMT) seem to progressively decrease with VE (Fig. 1). In contrast, for RV5 this trend is less marked and follows the seroresponse rate (Fig. 1). These dissimilarities could be explained by technical or interpretation differences of the RV-IgA ELISAs previously mentioned. An alternative explanation for cases in which RV-IgA seroresponse rates exceed VE against severe GE is that RV5 may be inducing serum RV-IgA without intestinal RV-IgA.73,82 This occurs in animals infected with some heterologous RV, for which the viral dose that induces antigenemia is lower than the dose that induces intestinal infection.73,82 Notably, the relationship between VE and VEI is specially discordant in trials in developing countries where VE and RV-IgA GMTs are in general lower and seroconversion rates in placebo recipients are higher than in developed countries (Fig. 1). This suggests that the low levels of RV-IgA detected in these countries are especially not protective in the placebo recipients. Maybe in these settings a threshold titer of RV-IgA is necessary to provide protection and seroconversions do not reflect the acquisition of this titer.
New Immune Markers as Candidate CoP for RV Vaccines
Further research should be encouraged to investigate new CoP for RV vaccines. According to EMA83: “When it is expected that cell-mediated immunity constitutes an important or even essential component of the overall immune response to an antigen, clinical studies to evaluate some type of cell-mediated immune correlates are encouraged." However, despite the important technological progress made in the past years in this field, measurement of B- and T-cell responses still faces many difficulties: standardization of cell preparation, timing of blood draw, cells conservation, reproducibility and inter-laboratory comparability, available volumes of blood in the pediatric population, and assay costs.84
The similar protection rates of RV1 and RV5 in countries where RV strains with high diversity of serotypes circulate suggest that immunity to RV is mostly heterotypic.73 Thus, the serotype specificity of the antibody response is probably a minor aspect of an ideal CoP. Efforts to identify better CoP should probably consider heterotypic markers that reflect intestinal immune responses; are capable of persisting in time; and whose measurement is not obstructed by maternal antibodies (serum IgG, serum NA, and milk stool antibodies).
There are 4 potential candidates for consideration:
RV- Secretory Ig (RV-SIg)
Polymeric antibodies (IgA or IgM) that have been secreted to the intestinal lumen can, by an unknown mechanism, be retro-transcytosed and reach the blood.4 They can be detected in serum by the presence of the secretory component, a part of the polymeric Ig receptor that is covalently attached to the Ig during the process of its secretion. One week after acute RV infection of children, RV-SIg was shown to be detectable in serum and to correlate with the amounts present in their duodenal fluid.85 Four months after viral infection RV-IgA persisted but RV-SIg had disappeared from the children's serum.86 In a small trial of a precursor to RV1 in which only seropositivity and not seroconversion was evaluated, we have shown that, like RV-IgA, these antibodies may correlate with protection when vaccines and placebo recipients are analyzed together.23 A drawback of measuring RV-SIg is that an important part seems to be composed of IgM, and it is uncertain if their presence can be a good marker of long term persisting immunity. Nevertheless, studies are underway to optimize the measurement of RV-SIg.
RV-specific antibody secreting cells (ASC) and memory B cells
In both animals and human studies, it has been shown that blood circulating, intestinally-induced ASC can reflect the intestinal antibody response.4 The measurement of human RV-ASC is challenging because of the small window (5–7 d) in which they can be measured and because in children most of them secrete IgM.4 Because it is not clear if RV-ASC can predict long-term persistence of immunity, efforts have concentrated on the study of RV-memory B cells (mBc). During an acute RV infection in children, circulating IgD- RV-mBc express intestinal-homing receptors (α4β7+, CCR9+), and thus probably reflect mucosal immunity.87 In a trial of the attenuated human RV1 vaccine precursor, when vaccines and placebo recipients were analyzed jointly, a very weak correlation was found between α4β7+, CCR9+ IgD- circulating RV-mBc and protection.75 Studies are underway to better understand and quantify RV-mBc.
RV- specific T cells
In animal models, it has been shown that most RV-IgA is dependent on CD4 T cells.88 Moreover, the development of RV-NA induced by vaccination both in animals3 and in children66 is dependent on the age of the individuals, that in its turn is correlated with the appearance of virus specific T cells.3 Similar to RV-mBc, in healthy adults RV-T cells express intestinal migration markers, suggesting that they may be reflecting intestinal RV-T cells.89 Most children with natural infection have undetectable or very low levels of RV-T cells measured by their capacity to secrete interferon gamma upon stimulation with antigen in vitro.90 Recent development of class II cell tetramers with RV-specific epitopes allowed detection of circulating RV-CD4 T cells that express intestinal homing receptors in healthy adults, and in children vaccinated with RIX4414.91 Further studies are necessary to explore their usefulness as correlate of protection.
Antibodies against other potentially protective heterotypic targets
A strategy to measure heterotypic antibodies to potentially protective antigens such as VP4 and VP7 in serum, that are not subject to the confounding effect of maternal IgG, is the measurement of IgA specific for either RV VP7 or VP4 proteins expressed in a system that conserves the neutralizing epitopes.50 Using this type of assay, predominantly homotypic IgG and IgA antibodies to both VP4 and VP7 have correlated with protection in adult challenge studies (Table 1).
In addition, antibodies against NSP4 (the viral enterotoxin) have also correlated with protection in the adult challenge studies and could also be a candidate CoP.50 A preliminary study has been performed in children to evaluate the response of vaccinees to different RV antigens in this system.92 Measurement of antibodies specific for recombinant RV proteins with the DELFIA technology is also a promising approach in this perspective.93
Finally, taking into account several recent papers94 95,96 that have shown RV-specific recognition of neutral oligosaccharides of the histo-blood group family, and antibodies that block binding of noroviruses to these receptors correlate with protection,97 it is important to determine if this is the case for RV.
Conclusions and Perspectives
The successful identification of CoP useful as a surrogate endpoint for VE studies depends on our understanding of the mechanism of protective immune response against natural RV infection. It is generally accepted that RV-NA against the infecting strain present in the intestine provides protection.6,43-45 In spite of this, protection following natural infection may not be solely correlated with the presence of type-specific RV-NA. Although quantitation of these antibodies in vaccinated individuals would probably be the best (mechanistic) CoP, it is impractical. Even though the presence of RV-specific IgA in stools (coproantibodies) would seem to be a way to measure these antibodies, technical issues and interference by maternal antibodies hamper the measurement of RV-NA or RV-IgA in stool samples.
Other than intestinal NA, current candidates for RV CoP are most probably non-mechanistic. Of these, the best candidate for a practically useful CoP is serum RV-IgA, since:
It reflects duodenal RV-IgA levels 4 months after RV natural infection.48
It correlates with protection after natural infections in children.58
It follows Prentice's first condition for a CoP as it correlates with the true clinical endpoint.25 It is, however, a relative CoP since the presence of RV-IgA does not always provide protection.12
Using meta-analysis it seems to correlate with protection in different vaccine settings for RV125, and for both RV1 and RV5 titers of RV-IgA seem to correlate with VE in different settings.24
The main drawbacks for the usefulness of RV-IgA as an immunological endpoint are:
It fails to fulfill Prentice's second condition for a surrogate endpoint, as it does not “fully capture the treatment's ”net effect “on the true clinical endpoint.” Its presence is “reasonably likely to predict clinical benefit” most probably classifying it as level 3 endpoint surrogate of protection.8
It seems to be a non-mechanistic CoP, hence, any vaccine change affecting this biomarker may or may not affect the clinical endpoint.
It does not seem to allow predicting individual protection and a dose effect (likelihood of not having a RV associated-GE with each 1 log increase in RV-IgA titer) has not been presented.25 Moreover, vaccinees without (less than 20 U/ml) serum RV-IgA have significantly less RV GE than placebo recipients,25 suggesting that factors other than serum RV-IgA play a role in protection.
Important challenges for the use of serum RV-IgA as a surrogate endpoint for RV vaccines are:
To use new approaches for the development of simpler and more reproducible assays to detect serum RV-IgA. This would ideally include standardization of the ELISA and of important key reagents, like the standard serum RV-IgA, for regulatory purposes.
To use other, or develop new, statistical methods to analyze all the information already collected in past RV VE trials.
A strategy to advance RV-IgA from a level 3 endpoint (a non-validated surrogate endpoint) toward a level 2 endpoint (a validated surrogate endpoint)8 could be implemented, based on the experience in the US for registration of new seasonal influenza vaccines, for pneumococcal conjugate vaccine, described above, and, following EMA's recommendations. In this context, evaluation of VE for new RV vacciness could be performed with a clinical endpoint (with delayed OPV), assessing serum RV-IgA with a standardize protocol and testing in parallel RV1 as a “control vaccine,” because usefulness of serum RV-IgA has been better established for this vaccine. If the correlation between RV-IgA and protection induced by new RV vaccines is similar to the one observed for RV1, serum RV-IgA could be considered a practical “validated” surrogate endpoint.
Our knowledge of the mechanisms of protection against RV in children seems incomplete and basic studies in this field would help identify other CoP. For the short term, serum RV-SIg seems like a good candidate, because it may give us an indirect measure of the immune response generated in the intestine.23 For the long term, circulating RV-mBc may prove to be a useful CoP, because they can reflect intestinal responses and provide a measure of the persistence of the response. Our poor understanding of the innate B cell response is an obstacle to this development.
Given the high burden of RV disease in developing countries and the rapid expansion in the number of countries introducing RV vaccines, the clinical development of new RV vaccine candidates using the classic Phase III efficacy approach will become more onerous and difficult to accomplish. Finding a RV CoP or surrogate marker(s) for clinical evaluation and the regulatory approval of the next generation of RV vaccines is now imperative, and requires interaction between the scientific and regulatory communities.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was fully funded by the World Health Organization.
References
- 1. Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:136-41; PMID:22030330; http://dx.doi.org/ 10.1016/S1473-3099(11)70253-5 [DOI] [PubMed] [Google Scholar]
- 2. Bhandari N, Rongsen-Chandola T, Bavdekar A, John J, Antony K, Taneja S, Goyal N, Kawade A, Kang G, Rathore SS, et al. . Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: a randomised, double-blind, placebo-controlled trial. Lancet 2014; 383:2136-43; PMID:24629994; http://dx.doi.org/ 10.1016/S0140-6736(13)62630-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Angel J, Franco MA, Greenberg HB. Rotavirus vaccines: recent developments and future considerations. Nat Rev Microbiol 2007; 5:529-39; PMID:17571094; http://dx.doi.org/ 10.1038/nrmicro1692 [DOI] [PubMed] [Google Scholar]
- 4. Franco MA, Angel J, Greenberg HB. Immunity and correlates of protection for rotavirus vaccines. Vaccine 2006; 24:2718-31; PMID:16446014; http://dx.doi.org/ 10.1016/j.vaccine.2005.12.048 [DOI] [PubMed] [Google Scholar]
- 5. Jiang B, Gentsch JR, Glass RI. The role of serum antibodies in the protection against rotavirus disease: an overview. Clin Infect Dis 2002; 34:1351-61; PMID:11981731; http://dx.doi.org/ 10.1086/340103 [DOI] [PubMed] [Google Scholar]
- 6. Ward RL, Clark HF, Offit PA. Influence of potential protective mechanisms on the development of live rotavirus vaccines. J Infect Dis 2010; 202 Suppl:S72-9; PMID:20684721; http://dx.doi.org/ 10.1086/653549 [DOI] [PubMed] [Google Scholar]
- 7. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Therap 2001; 69:89-95; PMID:11240971; http://dx.doi.org/ 10.1067/mcp.2001.113989 [DOI] [PubMed] [Google Scholar]
- 8. Fleming TR. Surrogate endpoints and FDA's accelerated approval process. Health Aff (Millwood) 2005; 24:67-78; PMID:15647217; http://dx.doi.org/ 10.1377/hlthaff.24.1.67 [DOI] [PubMed] [Google Scholar]
- 9. De Gruttola VG, Clax P, DeMets DL, Downing GJ, Ellenberg SS, Friedman L, Gail MH, Prentice R, Wittes J, Zeger SL. Considerations in the evaluation of surrogate endpoints in clinical trials. summary of a National Institutes of Health workshop. Control Clin Trials 2001; 22:485-502; PMID:11578783; http://dx.doi.org/ 10.1016/S0197-2456(01)00153-2 [DOI] [PubMed] [Google Scholar]
- 10. Qin L, Gilbert PB, Corey L, McElrath MJ, Self SG. A framework for assessing immunological correlates of protection in vaccine trials. J Infect Dis 2007; 196:1304-12; PMID:17922394; http://dx.doi.org/ 10.1086/522428 [DOI] [PubMed] [Google Scholar]
- 11. Plotkin SA, Gilbert PB. Nomenclature for immune correlates of protection after vaccination. Clin Infect Dis 2012; 54:1615-7; PMID:22437237; http://dx.doi.org/ 10.1093/cid/cis238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis 2008; 47:401-9; PMID:18558875; http://dx.doi.org/ 10.1086/589862] [DOI] [PubMed] [Google Scholar]
- 13. Follmann D. Augmented designs to assess immune response in vaccine trials. Biometrics 2006; 62:1161-9; PMID:17156291; http://dx.doi.org/ 10.1111/j.1541-0420.2006.00569.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chan IS, Li S, Matthews H, Chan C, Vessey R, Sadoff J, Heyse J. Use of statistical models for evaluating antibody response as a correlate of protection against varicella. Stat Med 2002; 21:3411-30; PMID:12407681; http://dx.doi.org/ 10.1002/sim.1268 [DOI] [PubMed] [Google Scholar]
- 15. Dunning AJ. A model for immunological correlates of protection. Stat Med 2006; 25:1485-97; PMID:16158409; http://dx.doi.org/ 10.1002/sim.2282 [DOI] [PubMed] [Google Scholar]
- 16. De Gruttola V, Fleming T, Lin DY, Coombs R. Perspective: validating surrogate markers–are we being naive? J Infect Dis 1997; 175:237-46; PMID:9203643; http://dx.doi.org/ 10.1093/infdis/175.2.237 [DOI] [PubMed] [Google Scholar]
- 17. Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med 1989; 8:431-40; PMID:2727467; http://dx.doi.org/ 10.1002/sim.4780080407 [DOI] [PubMed] [Google Scholar]
- 18. Gilbert PB, Qin L, Self SG. Evaluating a surrogate endpoint at three levels, with application to vaccine development. Stat Med 2008; 27:4758-78; PMID:17979212; http://dx.doi.org/ 10.1002/sim.3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Freedman LS, Graubard BI, Schatzkin A. Statistical validation of intermediate endpoints for chronic diseases. Stat Med 1992; 11:167-78; PMID:1579756; http://dx.doi.org/ 10.1002/sim.4780110204 [DOI] [PubMed] [Google Scholar]
- 20. Buyse M, Molenberghs G. Criteria for the validation of surrogate endpoints in randomized experiments. Biometrics 1998; 54:1014-29; PMID:9840970; http://dx.doi.org/ 10.2307/2533853 [DOI] [PubMed] [Google Scholar]
- 21. Andrews N, Borrow R, Miller E. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin Diagn Lab Immunol 2003; 10:780-6; PMID:12965904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siber GR. Methods for estimating serological correlates of protection. Dev Biol Stand 1997; 89:283-96; PMID:9272362 [PubMed] [Google Scholar]
- 23. Herrera D, Vasquez C, Corthesy B, Franco MA, Angel J. Rotavirus specific plasma secretory immunoglobulin in children with acute gastroenteritis and children vaccinated with an attenuated human rotavirus vaccine. Hum Vaccines Immunother 2013; 9:2409-17; PMID:23839157; http://dx.doi.org/ 10.4161/hv.25610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patel M, Glass RI, Jiang B, Santosham M, Lopman B, Parashar U. A systematic review of anti-rotavirus serum IgA antibody titer as a potential correlate of rotavirus vaccine efficacy. J Infect Dis 2013; 208:284-94; PMID:23596320; http://dx.doi.org/ 10.1093/infdis/jit166 [DOI] [PubMed] [Google Scholar]
- 25. Cheuvart B, Neuzil KM, Steele AD, Cunliffe N, Madhi SA, Karkada N, Han HH, Vinals C. Association of serum anti-rotavirus immunoglobulin A antibody seropositivity and protection against severe rotavirus gastroenteritis: analysis of clinical trials of human rotavirus vaccine. Hum Vaccines Immunother 2013; 10:505-11; PMID:24240068; http://dx.doi.org/ 10.4161/hv.27097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buyse M, Molenberghs G, Burzykowski T, Renard D, Geys H. The validation of surrogate endpoints in meta-analyses of randomized experiments. Biostatistics 2000; 1:49-67; PMID:12933525; http://dx.doi.org/ 10.1093/biostatistics/1.1.49 [DOI] [PubMed] [Google Scholar]
- 27. Daniels MJ, Hughes MD. Meta-analysis for the evaluation of potential surrogate markers. Stat Med 1997; 16:1965-82; PMID:9304767; http://dx.doi.org/ 10.1002/(SICI)1097-0258(19970915)16:17%3c1965::AID-SIM630%3e3.0.CO;2-M [DOI] [PubMed] [Google Scholar]
- 28. Salk JEMWJ, Francis T, Jr. A clinical, epidemiological and immunological evaluation of vaccination against epidemic influenza. Am J Hyg 1945; 42:57-93; PMID:45887724588772 [Google Scholar]
- 29. Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972; 70:767-77; PMID:4509641; http://dx.doi.org/ 10.1017/S0022172400022610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meiklejohn G, Kempe CH, Thalman WG, Lennette EH. Evaluation of monovalent influenza vaccines. II. Observations during an influenza a-prime epidemic. Am J Hyg 1952; 55:12-21; PMID:14885162 [DOI] [PubMed] [Google Scholar]
- 31. Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull 1979; 35:69-75; PMID:367490 [DOI] [PubMed] [Google Scholar]
- 32. Al-Khayatt R, Jennings R, Potter CW. Interpretation of responses and protective levels of antibody against attenuated influenza A viruses using single radial haemolysis. J Hyg (Lond) 1984; 93:301-12; PMID:6389697; http://dx.doi.org/ 10.1017/S0022172400064834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Jong JC, Palache AM, Beyer WE, Rimmelzwaan GF, Boon AC, Osterhaus AD. Haemagglutination-inhibiting antibody to influenza virus. Dev Biol 2003; 115:63-73; PMID:15088777 [PubMed] [Google Scholar]
- 34. Black S, Nicolay U, Vesikari T, Knuf M, Del Giudice G, Della Cioppa G, Tsai T, Clemens R, Rappuoli R. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J 2011; 30:1081-5; PMID:21983214; http://dx.doi.org/ 10.1097/INF.0b013e3182367662 [DOI] [PubMed] [Google Scholar]
- 35. Cox RJ. Correlates of protection to influenza virus, where do we go from here? Hum Vaccines Immunother 2013; 9:405-8; PMID:23291930; http://dx.doi.org/ 10.4161/hv.22908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Petrie JG, Ohmit SE, Johnson E, Cross RT, Monto AS. Efficacy studies of influenza vaccines: effect of end points used and characteristics of vaccine failures. J Infect Dis 2011; 203:1309-15; PMID:21378375; http://dx.doi.org/ 10.1093/infdis/jir015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eichelberger M, Golding H, Hess M, Weir J, Subbarao K, Luke CJ, Friede M, Wood D. FDA/NIH/WHO public workshop on immune correlates of protection against influenza A viruses in support of pandemic vaccine development, Bethesda, Maryland, US, December 10-11, 2007. Vaccine 2008; 26:4299-303; PMID:18582523; http://dx.doi.org/ 10.1016/j.vaccine.2008.06.012 [DOI] [PubMed] [Google Scholar]
- 38. Borrow R, Andrews N, Goldblatt D, Miller E. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect Immun 2001; 69:1568-73; PMID:11179328; http://dx.doi.org/ 10.1128/IAI.69.3.1568-1573.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med 1969; 129:1307-26; PMID:4977280; http://dx.doi.org/ 10.1084/jem.129.6.1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jodar L, Butler J, Carlone G, Dagan R, Goldblatt D, Kayhty H, Klugman K, Plikaytis B, Siber G, Kohberger R, et al. . Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine 2003; 21:3265-72; PMID:12804857; http://dx.doi.org/ 10.1016/S0264-410X(03)00230-5 [DOI] [PubMed] [Google Scholar]
- 41. Siber GR, Chang I, Baker S, Fernsten P, O'Brien KL, Santosham M, Klugman KP, Madhi SA, Paradiso P, Kohberger R. Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies. Vaccine 2007; 25:3816-26; PMID:17368878; http://dx.doi.org/ 10.1016/j.vaccine.2007.01.119 [DOI] [PubMed] [Google Scholar]
- 42. Reed GF, Meade BD, Steinhoff MC. The reverse cumulative distribution plot: a graphic method for exploratory analysis of antibody data. Pediatrics 1995; 96:600-3; PMID:7659485 [PubMed] [Google Scholar]
- 43. Estes MK, Kapikian AZ. Rotaviruses. In: Knipe DM, Howley PM, Griffin DE, R.A L, M.A. M, B. R, et al. , eds. Field's Virology: Lippincott Williams and Wilkins, 2007:1917-74. [Google Scholar]
- 44. Ward R. Mechanisms of protection against rotavirus infection and disease. Pediatr Infect Dis J 2009; 28:S57-9; PMID:19252425; http://dx.doi.org/ 10.1097/INF.0b013e3181967c16 [DOI] [PubMed] [Google Scholar]
- 45. Wood D. WHO informal consultation on quality, safety and efficacy specifications for live attenuated rotavirus vaccines Mexico City, Mexico, 8-9 February 2005. Vaccine 2005; 23:5478-87; PMID:16129525; http://dx.doi.org/ 10.1016/j.vaccine.2005.07.035 [DOI] [PubMed] [Google Scholar]
- 46. Ward RL, Bernstein DI, Shukla R, Young EC, Sherwood JR, McNeal MM, Walker MC, Schiff GM. Effects of antibody to rotavirus on protection of adults challenged with a human rotavirus. J Infect Dis 1989; 159:79-88; PMID:2535868; http://dx.doi.org/ 10.1093/infdis/159.1.79 [DOI] [PubMed] [Google Scholar]
- 47. Kapikian AZ, Wyatt RG, Levine MM, Yolken RH, VanKirk DH, Dolin R, Greenberg HB, Chanock RM. Oral administration of human rotavirus to volunteers: induction of illness and correlates of resistance. J Infect Dis 1983; 147:95-106; PMID:6296243; http://dx.doi.org/ 10.1093/infdis/147.1.95 [DOI] [PubMed] [Google Scholar]
- 48. Grimwood K, Lund JC, Coulson BS, Hudson IL, Bishop RF, Barnes GL. Comparison of serum and mucosal antibody responses following severe acute rotavirus gastroenteritis in young children. J Clin Microbiol 1988; 26:732-8; PMID:2835391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Green KY, Kapikian AZ. Identification of VP7 epitopes associated with protection against human rotavirus illness or shedding in volunteers. J Virol 1992; 66:548-53; PMID:1370092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yuan L, Honma S, Kim I, Kapikian AZ, Hoshino Y. Resistance to rotavirus infection in adult volunteers challenged with a virulent G1P1A[8]virus correlated with serum immunoglobulin G antibodies to homotypic viral proteins 7 and 4. J Infect Dis 2009; 200:1443-51; PMID:19785527; http://dx.doi.org/ 10.1086/606116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chiba S, Yokoyama T, Nakata S, Morita Y, Urasawa T, Taniguchi K, Urasawa S, Nakao T. Protective effect of naturally acquired homotypic and heterotypic rotavirus antibodies. Lancet 1986; 2:417-21; PMID:2874413; http://dx.doi.org/ 10.1016/S0140-6736(86)92133-1 [DOI] [PubMed] [Google Scholar]
- 52. O'Ryan ML, Matson DO, Estes MK, Pickering LK. Anti-rotavirus G type-specific and isotype-specific antibodies in children with natural rotavirus infections. J Infect Dis 1994; 169:504-11; PMID:8158022; http://dx.doi.org/ 10.1093/infdis/169.3.504 [DOI] [PubMed] [Google Scholar]
- 53. Ward RL, Clemens JD, Knowlton DR, Rao MR, Vanloon FPL, Huda N, Ahmed F, Schiff GM, Sack DA. Evidence that protection against rotavirus diarrhea after natural infection is not dependent on serotype-specific neutralizing antibody. J Infect Dis 1992; 166:1251-7; PMID:1331249; http://dx.doi.org/ 10.1093/infdis/166.6.1251 [DOI] [PubMed] [Google Scholar]
- 54. Zheng BJ, Lo SK, Tam JS, Lo M, Yeung CY, Ng MH. Prospective study of community-acquired rotavirus infection. J Clin Microbiol 1989; 27:2083-90; PMID:2550518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Coulson BS, Grimwood K, Masendycz PJ, Lund JS, Mermelstein N, Bishop RF, Barnes GL. Comparison of rotavirus immunoglobulin A coproconversion with other indices of rotavirus infection in a longitudinal study in childhood. J Clin Microbiol 1990; 28:1367-74; PMID:2166082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Matson DO, O'Ryan ML, Herera I, Pickering LK, Estes MK. Fecal antibody response to symptomatic and asymptomatic rotavirus infection. J Infect Dis 1993; 167:577-83; PMID:8440929; http://dx.doi.org/ 10.1093/infdis/167.3.577 [DOI] [PubMed] [Google Scholar]
- 57. Coulson BS, Grimwood K, Hudson IL, Barnes GL, Bishop RF. Role of coproantibody in clinical protection of children during reinfection with rotavirus. J Clin Microbiol 1992; 30:1678-84; PMID:1321167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Velazquez FR, Matson DO, Guerrero ML, Shults J, Calva JJ, Morrow AL, Glass RI, Pickering LK, Ruiz-Palacios GM. Serum antibody as a marker of protection against natural rotavirus infection and disease. J Infect Dis 2000; 182:1602-9; PMID:11069230; http://dx.doi.org/ 10.1086/317619 [DOI] [PubMed] [Google Scholar]
- 59. Velazquez FR, Matson DO, Calva JJ, Guerrero L, Morrow AL, Carter Campbell S, Glass RI, Estes MK, Pickering LK, Ruiz-Palacios GM. Rotavirus infections in infants as protection against subsequent infections. N Engl J Med 1996; 335:1022-8; PMID:8793926; http://dx.doi.org/ 10.1056/NEJM199610033351404 [DOI] [PubMed] [Google Scholar]
- 60. Clemens JD, Ward RL, Rao MR, Sack DA, Knowlton DR, van Loon FP, Huda S, McNeal M, Ahmed F, Schiff G. Seroepidemiologic evaluation of antibodies to rotavirus as correlates of the risk of clinically significant rotavirus diarrhea in rural Bangladesh. J Infect Dis 1992; 165:161-5; PMID:1309372; http://dx.doi.org/ 10.1093/infdis/165.1.161 [DOI] [PubMed] [Google Scholar]
- 61. Ward RL, Bernstein DI, Smith VE, Sander DS, Shaw A, Eiden JJ, Heaton P, Offit PA, Clark HF. Rotavirus immunoglobulin a responses stimulated by each of 3 doses of a quadrivalent human/bovine reassortant rotavirus vaccine. J Infect Dis 2004; 189:2290-3; PMID:15181577; http://dx.doi.org/ 10.1086/421248 [DOI] [PubMed] [Google Scholar]
- 62. Bernstein DI, Smith VE, Sherwood JR, Schiff GM, Sander DS, DeFeudis D, Spriggs DR, Ward RL. Safety and immunogenicity of live, attenuated human rotavirus vaccine 89-12. Vaccine 1998; 16:381-7; PMID:9607059; http://dx.doi.org/ 10.1016/S0264-410X(97)00210-7 [DOI] [PubMed] [Google Scholar]
- 63. Bernstein DI, Sack DA, Rothstein E, Reisinger K, Smith VE, O'Sullivan D, Spriggs DR, Ward RL. Efficacy of live, attenuated, human rotavirus vaccine 89-12 in infants: a randomised placebo-controlled trial. lancet 1999; 3547:287-90; ; http://dx.doi.org/ 10.1016/S0140-6736(98)12106-2 [DOI] [PubMed] [Google Scholar]
- 64. Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, Ngwira B, Victor JC, Gillard PH, Cheuvart BB, et al. . Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med 2010; 362:289-98; PMID:20107214; http://dx.doi.org/ 10.1056/NEJMoa0904797 [DOI] [PubMed] [Google Scholar]
- 65. Knowlton DR, Spector DM, Ward RL. Development of an improved method for measuring neutralizing antibody to rotavirus. J Virol Methods 1991; 33:127-34; PMID:1658027; http://dx.doi.org/ 10.1016/0166-0934(91)90013-P [DOI] [PubMed] [Google Scholar]
- 66. Ward RL, Kirkwood CD, Sander DS, Smith VE, Shao MY, Bean JA, Sack DA, Bernstein DI. Reductions in cross-neutralizing antibody responses in infants after attenuation of the human rotavirus vaccine candidate 89-12. J Infect Dis 2006; 194:1729-36; PMID:17109346; http://dx.doi.org/ 10.1086/509623 [DOI] [PubMed] [Google Scholar]
- 67. Armah GE, Breiman RF, Tapia MD, Dallas MJ, Neuzil KM, Binka FN, Sow SO, Ojwando J, Ciarlet M, Steele AD. Immunogenicity of the pentavalent rotavirus vaccine in African infants. Vaccine 2012; 30 Suppl 1:A86-93; PMID:22520142; http://dx.doi.org/ 10.1016/j.vaccine.2011.10.006 [DOI] [PubMed] [Google Scholar]
- 68. Ward RL, Knowlton DR, Zito ET, Davidson BL, Rappaport R, Mack ME. Serologic correlates of immunity in a tetravalent reassortant rotavirus vaccine trial. US Rotavirus Vaccine Efficacy Group. J Infect Dis 1997; 176:570-7; PMID:9291301; http://dx.doi.org/ 10.1086/514076 [DOI] [PubMed] [Google Scholar]
- 69. Bernstein DI, Smith VE, Sander DS, Pax KA, Schiff GM, Ward RL. Evaluation of WC3 rotavirus vaccine and correlates of protection in healthy infants. J Infect Dis 1990; 162:1055-62; PMID:2172394; http://dx.doi.org/ 10.1093/infdis/162.5.1055 [DOI] [PubMed] [Google Scholar]
- 70. Clark HF, Offit PA, Ellis RW, Eiden JJ, Krah D, Shaw AR, Pichichero M, Treanor JJ, Borian FE, Bell LM, et al. . The development of multivalent bovine rotavirus (strain WC3) reassortant vaccine for infants. J Infect Dis 1996; 174 Suppl 1:S73-80; PMID:8752294; http://dx.doi.org/ 10.1093/infdis/174.Supplement_1.S73 [DOI] [PubMed] [Google Scholar]
- 71. Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, Binka FN, Steele AD, Laserson KF, Ansah NA, et al. . Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:606-14; PMID:20692030; http://dx.doi.org/ 10.1016/S0140-6736(10)60889-6 [DOI] [PubMed] [Google Scholar]
- 72. Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, et al. . Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354:23-33; PMID:16394299; http://dx.doi.org/ 10.1056/NEJMoa052664 [DOI] [PubMed] [Google Scholar]
- 73. Angel J, Franco MA, Greenberg HB. Rotavirus immune responses and correlates of protection. Curr Opin Virol 2012; 2:419-25; PMID:22677178; http://dx.doi.org/ 10.1016/j.coviro.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Clark HF, Bernstein DI, Dennehy PH, Offit P, Pichichero M, Treanor J, Ward RL, Krah DL, Shaw A, Dallas MJ, et al. . Safety, efficacy, and immunogenicity of a live, quadrivalent human-bovine reassortant rotavirus vaccine in healthy infants. J Pediatr 2004; 144:184-90; PMID:14760258; http://dx.doi.org/ 10.1016/j.jpeds.2003.10.054 [DOI] [PubMed] [Google Scholar]
- 75. Rojas OL, Caicedo L, Guzman C, Rodriguez LS, Castaneda J, Uribe L, Andrade Y, Pinzón R, Narváez CF, Lozano JM, et al. . Evaluation of circulating intestinally committed memory B cells in children vaccinated with attenuated human rotavirus vaccine. Viral Immunol 2007; 20:300-11; PMID:17603846; http://dx.doi.org/ 10.1089/vim.2006.0105 [DOI] [PubMed] [Google Scholar]
- 76. Midthun K, Pang LZ, Flores J, Kapikian AZ. Comparison of immunoglobulin A (IgA), IgG, and IgM enzyme-linked immunosorbent assays, plaque reduction neutralization assay, and complement fixation in detecting seroresponses to rotavirus vaccine candidates. J Clin Microbiol 1989; 27:2799-804; PMID:2556433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ward RL, Bernstein DI. Lack of correlation between serum rotavirus antibody titers and protection following vaccination with reassortant RRV vaccines. US rotavirus vaccine efficacy group. Vaccine 1995; 13:1226-32; PMID:8578808; http://dx.doi.org/ 10.1016/0264-410X(95)00060-E [DOI] [PubMed] [Google Scholar]
- 78. Gonzalez R, Franco M, Sarmiento L, Romero M, Schael IP. Serum IgA levels induced by rotavirus natural infection, but not following immunization with the RRV-TV vaccine (Rotashield), correlate with protection. J Med Virol 2005; 76:608-12; PMID:15977224; http://dx.doi.org/ 10.1002/jmv.20404 [DOI] [PubMed] [Google Scholar]
- 79. Vesikari T, Karvonen A, Puustinen L, Zeng SQ, Szakal ED, Delem A, De Vos B. Efficacy of RIX4414 live attenuated human rotavirus vaccine in Finnish infants. Pediatr Infect Dis J 2004; 23:937-43; PMID:15602194; http:// dx.doi.org/ 10.1097/01.inf.0000141722.10130.50 [DOI] [PubMed] [Google Scholar]
- 80. Chan J, Nirwati H, Triasih R, Bogdanovic-Sakran N, Soenarto Y, Hakimi M, Duke T, Buttery JP, Bines JE, Bishop RF, et al. . Maternal antibodies to rotavirus: could they interfere with live rotavirus vaccines in developing countries? Vaccine 2011; 29:1242-7; PMID:21147127; http://dx.doi.org/ 10.1016/j.vaccine.2010.11.087 [DOI] [PubMed] [Google Scholar]
- 81. Patel M, Steele AD, Parashar UD. Influence of oral polio vaccines on performance of the monovalent and pentavalent rotavirus vaccines. Vaccine 2012; 30 Suppl 1:A30-5; PMID:22520134; http://dx.doi.org/ 10.1016/j.vaccine.2011.11.093 [DOI] [PubMed] [Google Scholar]
- 82. Feng N, Vo PT, Chung D, Vo TV, Hoshino Y, Greenberg HB. Heterotypic protection following oral immunization with live heterologous rotaviruses in a mouse model. J Infect Dis 1997; 175:330-41; PMID:9203654; http://dx.doi.org/ 10.1093/infdis/175.2.330 [DOI] [PubMed] [Google Scholar]
- 83. Agency EM. Note for Guidance on the Clinical Evaluation of Vaccines. In: EMEA , ed., 2005. [Google Scholar]
- 84. EMA Report from scientific workshop on serology assays and correlates of protection for influenza vaccines. 29 - 30 June 2010. EMA, Human Medicines Development and Evaluation; 2010. [Google Scholar]
- 85. Hjelt K, Grauballe PC, Schiotz PO, Andersen L, Krasilnikoff PA. Intestinal and serum immune response to a naturally acquired rotavirus gastroenteritis in children. J Pediatr Gastroenterol Nutr 1985; 4:60-6; PMID:2984402; http://dx.doi.org/ 10.1097/00005176-198502000-00012 [DOI] [PubMed] [Google Scholar]
- 86. Hjelt K, Grauballe PC, Andersen L, Schiotz PO, Howitz P, Krasilnikoff PA. Antibody response in serum and intestine in children up to six months after a naturally acquired rotavirus gastroenteritis. J Pediatr Gastroenterol Nutr 1986; 5:74-80; PMID:3944746; http://dx.doi.org/ 10.1097/00005176-198601000-00014 [DOI] [PubMed] [Google Scholar]
- 87. Jaimes MC, Rojas OL, Kunkel EJ, Lazarus NH, Soler D, Butcher EC, Bass D, Angel J, Franco MA, Greenberg HB. Maturation and trafficking markers on rotavirus-specific B cells during acute infection and convalescence in children. J Virol 2004; 78:10967-76; PMID:15452217; http://dx.doi.org/ 10.1128/JVI.78.20.10967-10976.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Franco MA, Greenberg HB. Immunity to rotavirus in T cell deficient mice. Virology 1997; 338:169-79; PMID:9400590 [DOI] [PubMed] [Google Scholar]
- 89. Rojas OL, Gonzalez AM, Gonzalez R, Perez-Schael I, Greenberg HB, Franco MA, Angel J. Human rotavirus specific T cells: quantification by ELISPOT and expression of homing receptors on CD4+ T cells. Virology 2003; 314:671-9; PMID:14554094; http://dx.doi.org/ 10.1016/S0042-6822(03)00507-5 [DOI] [PubMed] [Google Scholar]
- 90. Jaimes MC, Rojas OL, Gonzalez AM, Cajiao I, Charpilienne A, Pothier P, Kohli E, Greenberg HB, Franco MA, Angel J. Frequencies of virus-specific CD4(+) and CD8(+) T lymphocytes secreting gamma interferon after acute natural rotavirus infection in children and adults. J Virol 2002; 76:4741-9; PMID:11967291; http://dx.doi.org/ 10.1128/JVI.76.10.4741-4749.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Parra M, Herrera D, Calvo-Calle JM, Stern LJ, Parra-Lopez CA, Butcher E, Franco M, Angel J. Circulating human rotavirus specific CD4 T cells identified with a class II tetramer express the intestinal homing receptors alpha4beta7 and CCR9. Virology 2014; 452-453:191-201; PMID:24606696; http://dx.doi.org/ 10.1016/j.virol.2014.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yuan L, Ishida S, Honma S, Patton JT, Hodgins DC, Kapikian AZ, Hoshino Y. Homotypic and heterotypic serum isotype-specific antibody responses to rotavirus nonstructural protein 4 and viral protein (VP) 4, VP6, and VP7 in infants who received selected live oral rotavirus vaccines. J Infect Dis 2004; 189:1833-45; PMID:15122520; http://dx.doi.org/ 10.1086/383416 [DOI] [PubMed] [Google Scholar]
- 93. Kavanagh O, Zeng XL, Ramani S, Mukhopadhya I, Crawford SE, Kang G, Estes MK. A time-resolved immunoassay to measure serum antibodies to the rotavirus VP6 capsid protein. J Virol Methods 2013; 189:228-31; PMID:23183143; http://dx.doi.org/ 10.1016/j.jviromet.2012.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hu L, Crawford SE, Czako R, Cortes-Penfield NW, Smith DF, Le Pendu J, Estes MK, Prasad BV. Cell attachment protein VP8* of a human rotavirus specifically interacts with A-type histo-blood group antigen. Nature 2012; 485:256-9; PMID:22504179; http://dx.doi.org/ 10.1038/nature10996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Liu Y, Huang P, Jiang B, Tan M, Morrow AL, Jiang X. Poly-LacNAc as an age-specific ligand for rotavirus P[11]in neonates and infants. PLoS One 2013; 8:e78113; PMID:24244290; http://dx.doi.org/ 10.1371/journal.pone.0078113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ramani S, Cortes-Penfield NW, Hu L, Crawford SE, Czako R, Smith DF, Kang G, Ramig RF, Le Pendu J, Prasad BV, et al. . The VP8* domain of neonatal rotavirus strain G10P[11]binds to type II precursor glycans. J Virol 2013; 87:7255-64; PMID:23616650; http://dx.doi.org/ 10.1128/JVI.03518-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Reeck A, Kavanagh O, Estes MK, Opekun AR, Gilger MA, Graham DY, Atmar RL. Serological correlate of protection against norovirus-induced gastroenteritis. J Infect Dis 2010; 202:1212-8; PMID:20815703; http://dx.doi.org/ 10.1086/656364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Block SL, Vesikari T, Goveia MG, Rivers SB, Adeyi BA, Dallas MJ, Bauder J, Boslego JW, Heaton PM, Pentavalent Rotavirus Vaccine Dose Confirmation Efficacy Study Group . Efficacy, immunogenicity, and safety of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine at the end of shelf life. Pediatrics 2007; 119:11-8; PMID:17200266; http://dx.doi.org/ 10.1542/peds.2006-2058 [DOI] [PubMed] [Google Scholar]
- 99. Ciarlet M, Sani-Grosso R, Yuan G, Liu GF, Heaton PM, Gottesdiener KM, Arredondo JL, Schödel F. Concomitant use of the oral pentavalent human-bovine reassortant rotavirus vaccine and oral poliovirus vaccine. Pediatr Infect Dis J 2008; 27:874-80; PMID:18756184; http://dx.doi.org/ 10.1097/INF.0b013e3181782780 [DOI] [PubMed] [Google Scholar]
- 100. Vesikari T, Clark HF, Offit PA, Dallas MJ, DiStefano DJ, Goveia MG, Ward RL, Schödel F, Karvonen A, Drummond JE, et al. . Effects of the potency and composition of the multivalent human-bovine (WC3) reassortant rotavirus vaccine on efficacy, safety and immunogenicity in healthy infants. Vaccine 2006; 24:4821-9; PMID:16621194; http://dx.doi.org/ 10.1016/j.vaccine.2006.03.025 [DOI] [PubMed] [Google Scholar]
- 101. Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, Podder G, Vu DT, Le TP, Luby SP, et al. . Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:615-23; PMID:20692031; http://dx.doi.org/ 10.1016/S0140-6736(10)60755-6 [DOI] [PubMed] [Google Scholar]
- 102. Tregnaghi MW, Abate HJ, Valencia A, Lopez P, Da Silveira TR, Rivera L, Rivera Medina DM, Saez-Llorens X, Gonzalez Ayala SE, De León T, et al. . Human rotavirus vaccine is highly efficacious when coadministered with routine expanded program of immunization vaccines including oral poliovirus vaccine in Latin America. Pediatr Infect Dis J 2011; 30:e103-8; PMID:21378594 [DOI] [PubMed] [Google Scholar]
- 103. Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Cohen R, Meurice F, Han HH, Damaso S, Bouckenooghe A. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet 2007; 370:1757-63; PMID:18037080; http://dx.doi.org/ 10.1016/S0140-6736(07)61744-9 [DOI] [PubMed] [Google Scholar]
- 104. Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, et al. . Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354:11-22; PMID:16394298; http://dx.doi.org/ 10.1056/NEJMoa052434 [DOI] [PubMed] [Google Scholar]
- 105. Linhares AC, Velazquez FR, Perez-Schael I, Saez-Llorens X, Abate H, Espinoza F, López P, Macías-Parra M, Ortega-Barría E, Rivera-Medina DM, et al. . Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet 2008; 371:1181-9; PMID:18395579; http://dx.doi.org/ 10.1016/S0140-6736(08)60524-3 [DOI] [PubMed] [Google Scholar]