Figure 1.
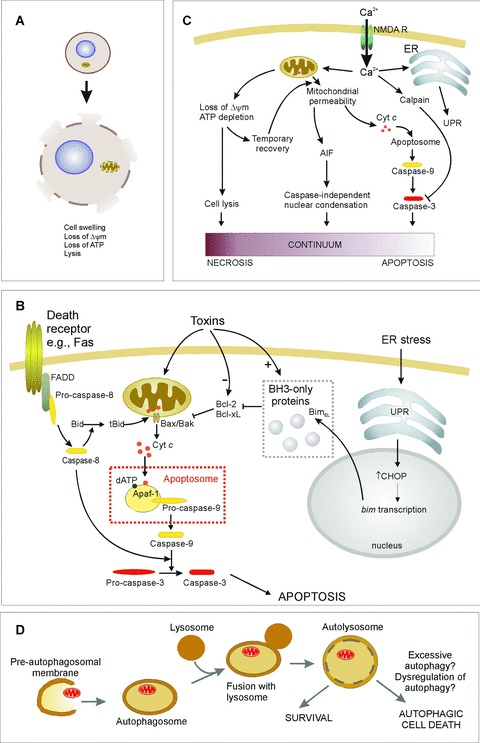
Mechanisms of cell death. See text for details. (A) Necrosis is characterized by loss of ATR which leads to cell lysis and release of cellular contents into the extracellular environment. (B) Apoptosis induction involves different pathways leading to caspase activation. In the extrinsic pathway ligand binding to death receptors {e.g. Fas) induces their trimerization and recruitment of caspase-8 through death domain-containing adaptor proteins (e.g. FADD). This leads to auto-activation of caspase-8, which can directly activate effector caspases. Alternatively in some cells Bid cleavage by caspase-8 is required for death. Truncated Bid translocates to the mitochondria and activates the intrinsic pathway. The intrinsic pathway is activated by toxins or conditions that cause stress to the mitochondria or endoplasmic reticulum (ER). Such stresses often alter the levels of, or cause post-translational modification of, Bcl-2 family proteins, initiating the release of cytochrome c from the mitochondria. For example, ER stress up-regulates the unfolded protein response (UPR), which increases expression of BimEL, a BH3-only protein that antagonizes Bcl-2 and Bcl-xL, permitting Bax-mediated cytochrome c release. Once in the cytosol, cytochrome c stimulates formation of the apoptosome complex, leading to caspase-9 activation. Initiator caspases (e.g. caspase-8 and -9) are shown in yellow, downstream effector caspases (e.g. caspase-3) are shown in red. (C) Excitotoxicity is caused by the influx of Ca2+ through the NMDA receptor. This can result in apoptosis, necrosis, or cell death that exhibits apoptotic nuclear morphology without caspase activation, suggesting a continuum of death patterns. (D) Autophagic cell death may be due to excessive autophagic digestion, as a result of dysregulation of the pathway by unknown mechanisms.
