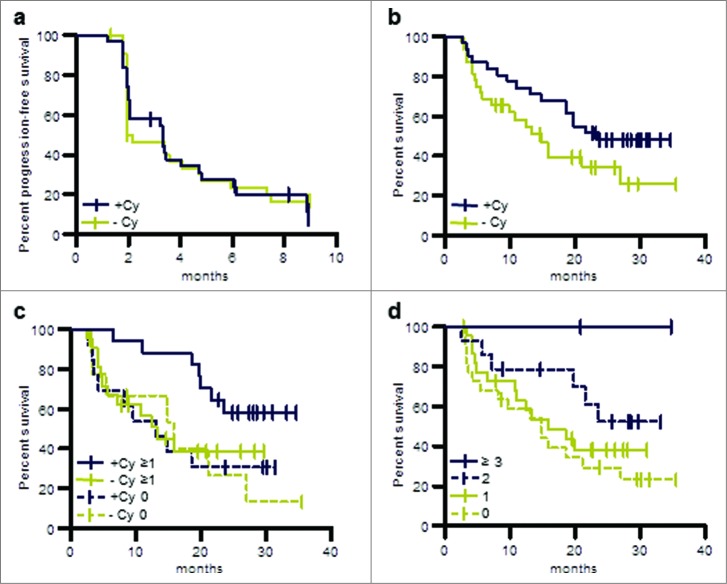
Figure 7.
OS and PFS of patients treated in the phase II study (per protocol population). (a) PFS of patients pre-treated with cyclophosphamide or without such pre-treatment. (b) OS of patients pre-treated with cyclophosphamide or without such pre-treatment. (c) OS of patients evaluable for immune responses in the following groups: immune responders pre-treated with cyclophosphamide, +Cy ≥ 1; patients pre-treated with cyclophosphamide for whom no immune response was observed, +Cy 0; immune responders without cyclophosphamide pre-treatment, −Cy ≥ 1; patients without cyclophosphamide pre-treatment for whom no immune response was observed, −Cy 0. (d) OS of patients for whom no immune response was observed, immune responders to one TUMAP, immune responders to 2 TUMAPs or immune responders to at least 3 TUMAPs.