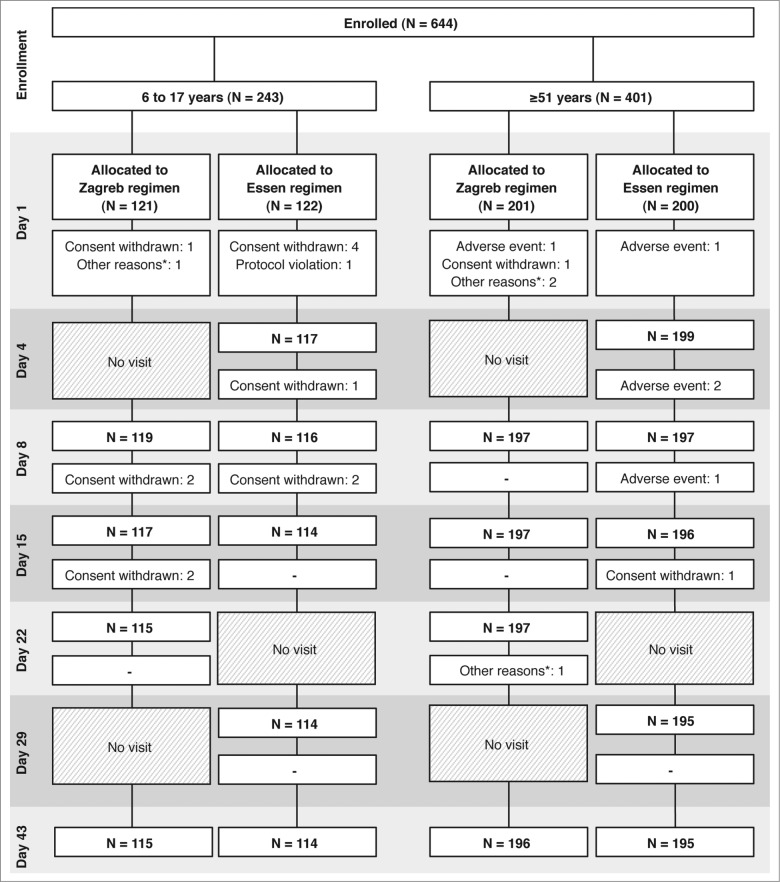
Figure 1.
Flow diagram of the trial. *Other reasons included that the subject went out (2 subjects at Day 1, both in the Zagreb regimen, 1 in the 6 to 17 years cohort, the other in the ≥51 years cohort); screening failure (1 subject at Day 1, in the Zagreb regimen, and in the ≥51 years cohort); and withdrawal of consent for continuing study participation (1 subject at Day 22 in the Zagreb regimen, and in the ≥51 years cohort).