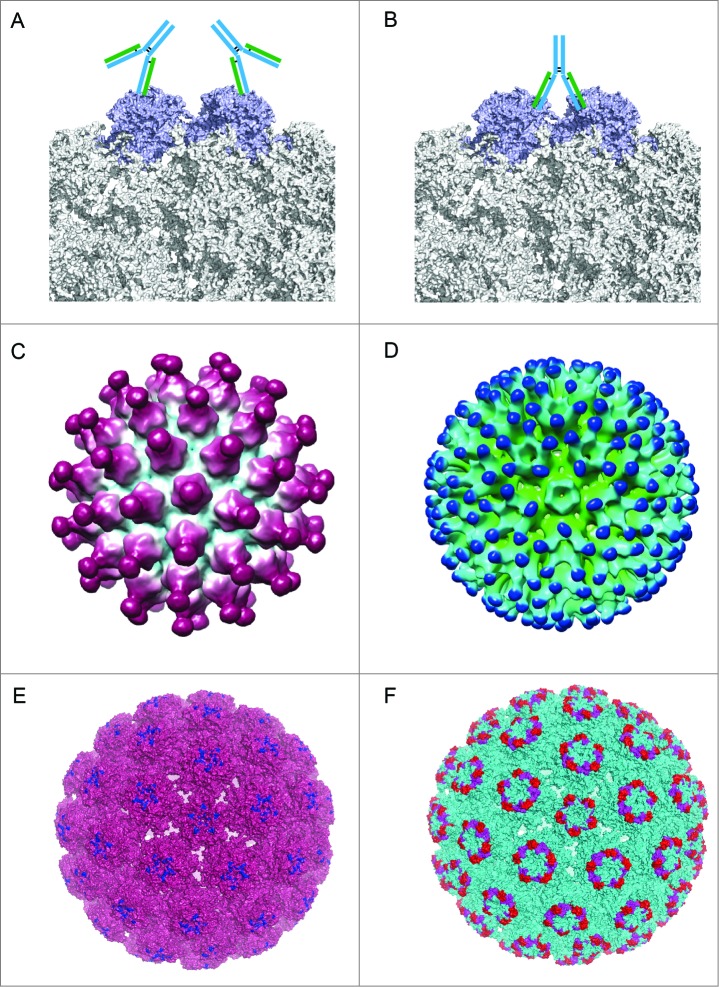
Figure 5.
Different binding patterns of antibodies to BPV and HPV.19,102 (A) mAb #9 binding to the outer surface of the hexavalent capsomere. (B) mAb 5B6 binding to the 2 L1 molecules of the adjacent hexavalent capsomeres. (C) A 3D reconstruction structure of mAb H11.B2 binding to HPV11 VLP (the DE loops on L1, Fig. 4) indicates that the binding sites are located at the center of the capsomere. (D) A 3D reconstruction structure based on cryo-EM data of mAb H16.V5 binding to HPV16 VLP (FG and HI loops on L1, Fig. 4) shows that H16.V5 only binds to the hexavalent capsomeres but not the pentavalent capsomeres.19 Recently, atomic model of the V5 epitope were built with higher resolution 3D reconstruction of cryo-EM data, demonstrating certain conformational changes induced by V5 binding to its epitope and confirming the preferential binding to the hexavalent capsomeres.151 The different binding models demonstrate different neutralization mechanisms for neutralizing antibodies exerting their effects against the viral infection. (E) The 3D structural model of mAb H11.B2 binding to HPV VLP in which the binding site of H11.B2 was indicated in blue and warmpink for VLP. (F) The 3D structural model of mAb H16.V5 binding to HPV16 VLP, with magenta for H16.V5 binding site on FG loop and red on HI loop, and cyan for VLP.