Abstract
The Italian National Plan of Measles and Rubella Elimination 2010–2015 has deferred the objective to reduce congenital rubella syndrome (CRS) to <1 case per 100 000 live births to 2015 and has highlighted the need to reduce to <5% susceptibility to rubella among women in childbearing-age. In Puglia region, MMR vaccine coverage is 93% in newborns (cohort 2010; one dose), 85% in children 5–6 years old and 77% in adolescents (cohort 2005 and 1997, respectively; two doses). Combining available seroepidemiological data and results of a survey on the attitude towards rubella vaccination and rubella testing before pregnancy, we could estimate that 5.7% of Apulian women in childbearing-age are currently susceptible to rubella infection. The regional infectious disease routine notification system reported no cases of CRS and rubella in pregnancy in 2001–2010 period. The inconsistency among the mentioned data triggered the evaluation of the reliability of disease reporting. We performed a retrospective case-finding for the years 2003–2011. We scanned the regional hospital discharge registry to identify hospitalizations for rubella in pregnancy and CRS and retrieve individual records. We also searched for clinical history of CRS mothers in the delivery assistance certificate registry. We identified one CRS, two confirmed and four suspected congenital infections, and seven cases of rubella in pregnancy. Passive surveillance of CRS and rubella in pregnancy appears not to be reliable in the light of strengthening rubella elimination strategies.
Keywords: Rubella Syndrome, Congenital Rubella, Pregnancy, Rubella vaccine, Surveillance system, Italy
Background
Rubella is usually a mild viral infection that can remain sub-clinic in up to 50% of cases. Infection contracted during pregnancy, particularly during the first trimester, can be vertically transmitted to the fetus resulting in miscarriage, stillbirth or in a range of physical malformations, known as congenital rubella syndrome (CRS).1
The elimination of rubella (<1 case per million inhabitants) and the control of CRS (<1 case per 100 000 live births) by 2015 are public heath priorities for Europe.2,3 As of December 2012, all European countries had introduced rubella-containing vaccines in their routine immunization programmes, even with differences in the timing of implementation, vaccination strategies and level of coverage achieved.4
An epidemiological assessment conducted in 2008 among the 32 EUVAC.NET (European surveillance network for vaccine-preventable diseases) participating countries revealed that in 24/28 countries that had established mandatory notification system for rubella, the median annual incidence of cases had decreased from 7.2 to 0.3 per million inhabitants in the period 2000–2008. Despite the overall reduction in the number of cases, since 2000 several European countries have experienced large outbreaks of rubella.5-10
In Italy, the global goal of rubella elimination and CRS prevention by the year 2007 has been first defined in the National Plan of Measles and Congenital Rubella Elimination 2003–2007 (PNEMoRC).11 Even though the notification of rubella cases became mandatory in 1970, the congenital rubella (CR) reporting system has been mandatory only for the five-year period 1987–1991 and no surveillance for rubella in pregnancy has ever been implemented before January 2005, when both CR and rubella in pregnancy became statutory notifiable as recommended by the PNEMoRC.12,13
As in 2007 the elimination goal has not been reached, the new PNEMoRC 2010–2015 has deferred its achievement to the year 2015 and has highlighted the need to strengthen the surveillance and reduce to less than 5% susceptibility to rubella among women in childbearing-age (15–49 y).3,14
According to a seroprevalence study conducted in Italy in 2004, 91.9% of women older than 15 y were positive for rubella antibodies.15 The Italian Behavioral Risk Factor Surveillance System (PASSI) revealed that in the 2009–2012 period, 34% of women in childbearing-age had an unknown rubella immune status and 2% had a negative rubella test. The highest vaccination coverage was reported among women aged 18–24 y (57%), having been the target of catch-up campaign promoted by PNEMoRC 2003–2007.16
In Puglia region (Southern Italy), vaccination coverage for one dose of measles-mumps-rubella (MMR) was 93% in newborns (birth cohort 2010), 85% and 77% for two doses in children aged 5–6 y (cohort 2005) and adolescents (cohort 1997), respectively. Vaccination uptake reached 70.9% for one MMR dose and 49.8% for two doses in 1991–1997 birth cohorts, during the supplementary vaccination campaign conducted between 2004 and 2006 in accordance with PNEMoRC 2003–2007.17 PASSI data showed that in Puglia, in the 2008–2011 period, 59.4% of women between 18 and 49 y were not susceptible to rubella (vaccinated or IgG positive), 38% had an unknown rubella immune status and 2.6% had a negative rubella test (unpublished data). Considering the number of women in childbearing-age in Puglia (n = 894 760, Italy’s National Census Bureau - ISTAT estimate), those having an unknown rubella immune status should amount to about 340 000 (38%). Applying nationally available seoroprevalence data (8.1% of 340 000 women) and adding those with a negative rubella test (2.6% of 894 760), it can be estimated that 5.7% of Apulian women in childbearing-age are susceptible to rubella infection, still above the threshold for disease elimination.
Even though data reported to the Apulian infectious disease routine notification system for the period from 2001 to 2012 showed a median annual incidence of 2.2 × 100 000 with a peak of rubella cases both in 2002 and 2008, no cases of CR and rubella in pregnancy were reported.
In the light of further efforts to be implemented in PNEMoRC 2010–2015, the inconsistency among the prevalence of susceptible women and the persistency of rubella virus circulation triggered the need of evaluating the reliability of the regional notification system of CR and rubella in pregnancy, in order to strengthen surveillance activities and monitor the progress in the achievement of the elimination goal by 2015.
Results
We identified 14 hospital discharge records coded for CR and none for rubella in pregnancy. The linkage between HDR and DACR enabled the identification of 13/14 mothers. One mother was not traceable in the DACR due to no legal recognition of the newborn. We retrieved the individual hospital records for 13 newborns and 12 mothers.
Clinical manifestations of CR were recorded in two IHRs: the first case presented with loss of hearing, cataract, congenital cardiopathy, peripheral pulmonary stenosis and cerebral anomalies at ultrasound; the second case presented with meningo-encephalitis and thrombocytopenia. Rubella test was available in the IHR of six newborns: three cases were IgM and IgG positive; two cases were IgG positive only; one case was PCR positive.
An anamnesis for rubella in pregnancy was reported in five IHRs. Rubella test was available in the IHRs of five pregnant women: four of them had positive rubella-specific IgM titre dated during the gestational period; one woman had a rubella IgG positive test dated at the delivery (CR clinical manifestations in the newborn drove physicians to require the immunological status of the mother).
No data suggesting rubella infection were recorded for 6/13 newborns and the respective mothers.
Our final classification of confirmed cases was: one congenital rubella syndrome, two congenital rubella infections (CRI) and six rubella infections in pregnancy. Rubella IgM positivity in the no legally recognized newborn suggested the suspect of rubella infection in pregnancy in the respective mother (Table 1).
Table 1. Rubella in pregnancy and CR. Information recorded and classification of cases. Puglia region, Italy, 2003–2011.
| Event | Clinical signs in the newborn | Positive test* in the newborn | Positive anamnesis of the mother | Classification of the newborn | Classification of the mother |
|---|---|---|---|---|---|
| 1 | Yes | Yes | N.A. | CRS | Rubella in pregnancy |
| 2 | Yes | Yes | Yes | CRI | Rubella in pregnancy |
| 3 | No | Yes | Yes | CRI | Rubella in pregnancy |
| 4 | No | No | Yes | Suspect | Rubella in pregnancy |
| 5 | No | No | Yes | Suspect | Rubella in pregnancy |
| 6 | No | No | Yes | Suspect | Rubella in pregnancy |
| 7 | No | Yes | N.A. | Suspect | Suspect |
IgM and/or PCR.
Discussion
In Italy, between 2005 and 2012, a total of 91 confirmed cases of rubella in pregnancy, three probable cases and 48 laboratory-confirmed cases of CRS were reported to the national surveillance system.18 Our findings reveal that rubella in pregnancy and congenital rubella infection continue to represent a public health threat also in Puglia region. Despite the efforts in achieving high level of vaccination coverage in children and adolescents, the proportion of women in childbearing-age immune to rubella is still low (about 60%) with an estimate of 5.7% of them susceptible to the infection. One case of congenital rubella syndrome and two of congenital rubella infection were traced in 2008 during the peak of disease incidence in Italy.5
Our study brings to light the problem of under-reporting and the capability of the infectious disease notification system in identifying rubella in pregnancy and CR cases. None of the cases tracked in the retrospective case-finding had been notified through the enhanced surveillance system that could be limited as unique source of information on such diseases. Further efforts are required to strengthen the surveillance in this phase of rubella elimination and CRS control.14 Information gaps might be bridged trough an active integration with data available in alternative sources, as showed in our paper.
The methodology we used for the retrospective case-finding was simple to apply, repeatable and effective; however, factors related with the type of data source or the nature of the disease might affect the results. First, information recorded in the Individual Hospital Records can be no exhaustive or create mis-interpretation whether missing data really correspond to a “no case” or depend by the malpractice of the health care workers in charge of drawing the medical history of the patient. Second, due to the nature of the disease, some clinical manifestations of CRS can arise later in life and consequently they are not diagnosed at birth or before the newborn is discharged. In addition, to confirm the infection in the newborn, the persistence of rubella IgG should be checked between six and 12 mo of age.5,19,20 Finally, our methodology does not collect information on the follow-up of suspected CR cases, crucial to formulate a final classification. Another possible reason of underestimation is that hospitalization is rarely required for rubella in pregnancy cases.
The need of active case-finding for an effective surveillance of CRS has been already discussed by Muscat el al., underlining the importance of a multidisciplinary approach that includes the participation of specialists involved in the care of pregnant women and newborns.5 Our findings highlight the importance to establish on a routine basis the review of alternative sources to the passive surveillance system for CR case-finding. The periodical feedback of epidemiological results to health care workers involved in the surveillance might help to increase the awareness of the disease and encourage case notification.
Another crucial point towards the elimination goal is the role played by general practitioners, health personnel and specialists involved in women’s health that should provide with pre-conception counseling and promote vaccination to reduce the pocket of susceptible to rubella to less than 5%. In Puglia region, PASSI survey showed that 41% of women aged 18–49 y are not vaccinated and have either a negative rubella test or unknown immune status, suggesting a low level of awareness among women of childbearing-age about the risk of the disease contracted during the pregnancy. This evidence, even though in Italy rubella test is recommended and offered free of charge to all females as pre-conception screening since 1998 and rubella vaccination is actively offered to susceptible fertile women taking advantage of all opportunities of encounter (i.e., the anti-tetanus-diphteria-pertussis booster dose, the anti-HPV immunization session, the first invitation for the pap-test screening, the visit at a travel medicine service, promptly after delivery or after spontaneous or induced abortion) since 2005.21,22
To achieve and maintain the elimination goal, more efforts are needed, at subnational administrative level, to reach and sustain vaccination coverage above 95% with at least one dose of rubella vaccine in the general population. In order to interrupt endemic rubella transmission and to reduce susceptible populations, more appropriate strategies and supplementary immunization activities should be focused on women of childbearing-age and vulnerable groups with suboptimal vaccination coverage, including students attending high school or universities, military, academic and health care personnel.22,23
Our study confirms that the prevention of rubella still represent a priority for public health professionals. In an era when effective preventive measures like rubella vaccine are easily accessible for the general population, health threats as rubella in pregnancy and congenital rubella are no longer ethically tolerable. Our model of case-finding together with other evidence-based surveillance actions, such as the establishment of a rubella test negative laboratory-based reporting system, could strengthen disease control towards the elimination.
Methods
In 2013 we conducted a retrospective case-finding of CR and rubella in pregnancy in Puglia region.
Data sources
For the purpose of this study we used alternative data sources to routine notification system:
Hospital Discharge Registry (HDR), that collects data on discharge diagnoses (one main and up to five secondary diagnoses) and procedures of all patients admitted to hospitals in the region. Discharge diagnosis and procedures are coded using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD9-CM).
Delivery Assistance Certificate Registry (DACR) that collects data on maternal-child health at the delivery, providing information on socio-demographic characteristics of the mother and on the health status of the newborn.
Individual Hospital Records (IHRs), available on demand from the hospitals, that systematically collect medical history and care provided to a single patient during hospitalization. Medical information is recorded by health care professionals (physicians or nurses) and has legal value.
According to the availability of selected sources, we performed a retrospective case-finding for the period 2003–2011. Information collected from each data source is listed in Table 2.
Table 2. Rubella in pregnancy and CR. Information collected in different data sources. Puglia region, Italy, 2003–2011.
| Variable | HDR | DACR | IHR |
|---|---|---|---|
| Demographic data (DoB, sex, residency) | + | + | + |
| Personal ID number | + | + | + |
| Weight at birth | + | + | + |
| ICD9-CM code of main and secondary diagnosis | + | + | |
| Date of hospitalization and discharge | + | + | |
| Hospital code | + | + | + |
| Number of Individual hospital record | + | + | |
| Gestational week at delivery | + | + | + |
| Anamnesis | + | ||
| Clinical information | + | ||
| Laboratory/diagnostic test results | + |
Procedures
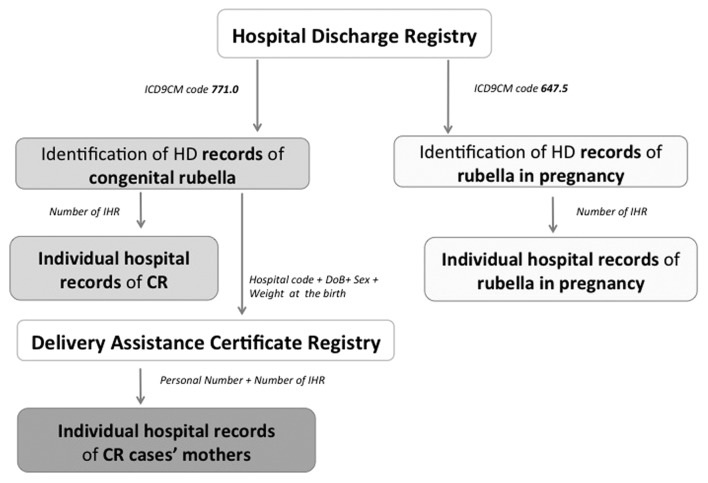
We reviewed the regional Hospital Discharge Registry to identify hospitalizations with discharge code of rubella in pregnancy (ICD9-CM code: 647.5) or congenital rubella (ICD9-CM code: 771.0). We scanned across discharge diagnoses in each record for any mention of these disease codes and we retrieved the IHR of relative pregnant women and newborns. We identified the mothers of CR patients linking HDR of the newborns with DACR by using the variables “date of birth + sex + weight at the birth + hospital code” as unique key. We searched the hospital discharge record of each CR cases’ mother by using their own personal ID number and thus identifying individual hospital record number to be retrieved (Fig. 1). When all IHRs of rubella in pregnancy cases, congenital rubella cases and their respective mothers were obtained, we gathered data on anamnesis, clinical history and laboratory results. We classified cases by applying the criteria for rubella infection and congenital rubella stipulated by EU Commission Decision of 8 August 2012.19 For those mothers whose rubella test was not documented but the information on rubella in pregnancy was recorded by the physician providing care, we classified the case of rubella in pregnancy as confirmed, according with the Italian legislation on legality of IHR.24
Figure 1. Flowchart of rubella in pregnancy and CR case-finding procedures. Puglia region, Italy, 2003–2011.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We acknowledge Giovanni Caputi, Maria Stella Bianco, Vincenzo Coviello, Antonio Daleno, Giovanni Guario, Alessandro Guaccero, Anna Melcarne, Giuseppe Piano, Giuseppe Spagnuolo, and Cristina Sponsilli, who collaborated in the collection of Individual Hospital Records from hospital archives region-wide. We thank Paolo Trerotoli and Nicola Bartolomeo who supported us in the analysis of Delivery Assistance Certificate Registry. We also thank Biagio Pedalino and Cristina Giambi for their suggestions for the manuscript.
Glossary
Abbreviations:
- CR
Congenital Rubella
- CRI
Congenital Rubella Infection
- CRS
Congenital Rubella Syndrome
- DACR
Delivery Assistance Certificate Registry
- EUVAC.NET
European surveillance network for vaccine-preventable diseases
- HDR
Hospital Discharge Registry
- ICD9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- IHR
Individual Hospital Record
- MMR
Measles, Mumps, Rubella vaccine
- N.A.
Not available
- PNEMoRC
Piano Nazionale di Eliminazione del Morbillo e della Rosolia Congenita - National Plan of Measles and Congenital Rubella Elimination
- PASSI
Progressi delle Aziende Sanitarie per la Salute in Italia - Italian Behavioral Risk Factor Surveillance System
References
- 1.Centers for Disease Control and Prevention (CDC).. Control and prevention of rubella: evaluation and management of suspected outbreaks, rubella in pregnant women, and surveillance for congenital rubella syndrome. MMWR Recomm Rep 2001; 50:RR-121 - 23; PMID: 11475328 [PubMed] [Google Scholar]
- 2.World Health Organization (WHO). Strategic plan for measles and congenital rubella infection in the European Region of WHO. Copenhagen: WHO Regional Office for Europe; 2003. Available at: http://www.euro.who.int/__data/assets/pdf_file/0020/79022/E81567.pdf
- 3.World Health Organization (WHO). WHO Regional Committee for Europe resolution EUR/RC60/R12 on renewed commitment to elimination of measles and rubella and prevention of congenital rubella syndrome by 2015 and sustained support for polio-free status in the WHO European Region. Copenhagen: WHO Regional Office for Europe; 2010. Available at: http://www.euro.who.int/__data/assets/pdf_file/0016/122236/RC60_eRes12.pdf
- 4.Centers for Disease Control and Prevention (CDC).. Rubella and congenital rubella syndrome control and elimination - global progress, 2000-2012. MMWR Morb Mortal Wkly Rep 2013; 62:983 - 6; PMID: 24304830 [PMC free article] [PubMed] [Google Scholar]
- 5.Muscat M, Zimmerman L, Bacci S, Bang H, Glismann S, Mølbak K, Reef S, EUVAC.NET group.. Toward rubella elimination in Europe: an epidemiological assessment. Vaccine 2012; 30:1999 - 2007; http://dx.doi.org/ 10.1016/j.vaccine.2011.12.016; PMID: 22178098 [DOI] [PubMed] [Google Scholar]
- 6.Rafila A, Marin M, Pistol A, Nicolaiciuc D, Lupulescu E, Uzicanin A, Reef S.. A large rubella outbreak, Romania--2003. Euro Surveill 2004; 9:7 - 9; PMID: 15192257 [DOI] [PubMed] [Google Scholar]
- 7.Revello MG, Gorini G, Zavattoni M, Furione M, Gerna G.. Congenital rubella infection following rubella outbreak in northern Italy, 2002: need for an effective vaccination programme. Eur J Clin Microbiol Infect Dis 2004; 23:780 - 3; http://dx.doi.org/ 10.1007/s10096-004-1213-6; PMID: 15368099 [DOI] [PubMed] [Google Scholar]
- 8.García L, Red de Vigilancia Epidemiológica de la Comunidad de Madrid (Epidemiological Surveillance Network of the Autonomous Region of Madrid).. Outbreak of rubella in the Madrid region, Spain, 2005. Euro Surveill 2005; 10:E050707.2; PMID: 16785654 [DOI] [PubMed] [Google Scholar]
- 9.Hahné S, Macey J, van Binnendijk R, Kohl R, Dolman S, van der Veen Y, Tipples G, Ruijs H, Mazzulli T, Timen A, et al.. Rubella outbreak in the Netherlands, 2004-2005: high burden of congenital infection and spread to Canada. Pediatr Infect Dis J 2009; 28:795 - 800; http://dx.doi.org/ 10.1097/INF.0b013e3181a3e2d5; PMID: 19710586 [DOI] [PubMed] [Google Scholar]
- 10.Paradowska-Stankiewicz I, Czarkowski MP, Derrough T, Stefanoff P.. Ongoing outbreak of rubella among young male adults in Poland: increased risk of congenital rubella infections. Euro Surveill 2013; 18:20485; PMID: 23725976 [PubMed] [Google Scholar]
- 11.Ministero della Salute. Italy (2003). Piano nazionale per l'eliminazione del morbillo e della rosolia congenita 2003-2007. Conferenza Stato-Regioni del 13 novembre 2003. Italian. Available at: http://www.governo.it/backoffice/allegati/20894-1712.pdf
- 12.Ciofi degli Atti M, Filia A, Verteramo R, Iannazzo S, Curtale F, Masini L, De Santis M, Pompa MG.. First cases of rubella infection during pregnancy detected by new reporting system in Italy. Euro Surveill 2006; 11:E060323.5; PMID: 16804234 [DOI] [PubMed] [Google Scholar]
- 13.Ministero della Salute. Italy (2004). Notifica obbligatoria della sindrome/infezione da rosolia congenita. G.U. Serie Generale, n. 259 del 4 novembre 2004. Italian. Available at: http://www.epicentro.iss.it/problemi/rosolia/Decreto.pdf
- 14.Ministero della Salute. Italy (2011). Piano nazionale per l'eliminazione del morbillo e della rosolia congenita 2010-2015. Intesa Stato-Regioni del 23 marzo 2011. Italian. Available from: http://www.salute.gov.it/imgs/C_17_pubblicazioni_1519_allegato.pdf
- 15.Rota MC, Bella A, Gabutti G, Giambi C, Filia A, Guido M, De Donno A, Crovari P, Ciofi Degli Atti ML, Serological Study Group.. Rubella seroprofile of the Italian population: an 8-year comparison. Epidemiol Infect 2007; 135:555 - 62; http://dx.doi.org/ 10.1017/S0950268806007400; PMID: 17076939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Istituto Superiore di Sanità. Italy 2012. Progressi delle Aziende Sanitarie per la Salute in Italia: la sorveglianza Passi (PASSI). Italian. Available at: http://www.epicentro.iss.it/passi/dati/VaccinazioneAntirosolia.asp
- 17.Commissione tecnico-scientifica regionale vaccini, Apulia Region, Italy (2012). Piano nazionale per l’eliminazione del morbillo e della rosolia congenita (PNEMoRc) 2010-2015: Accordo Stato-Regioni e Province Autonome, Rep. Atti n. 66/CSR. Recepimento e adozione del correlato Piano regionale. Deliberazione Della Giunta Regionale 7 agosto 2012, n. 1600. Bollettino Ufficiale della Regione Puglia - n. 132 dell’11-09-2012. Italian. Available at: http://www.regione.puglia.it/index.php?page=burp&opz=getfile&file=o-3.htm&anno=xliii&num=132
- 18.Giambi C, Filia A, Rota MC, Bella A, Declich S. Vaccinare le donne in età fertile suscettibili alla rosolia: ogni occasione è buona! Epicentro 2013. Italian. Available at: http://www.epicentro.iss.it/approfondimenti/2013/8marzoVaccinazioneRosolia.asp
- 19.Commission Implementing Decision 2012/506/EU of 8 August 2012, amending Decision 2002/253/EC laying down case definitions for reporting communicable diseases to the Community network under Decision No 2119/98/EC of the European Parliament and of the Council. Available at: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2012:262:0001:0057:EN:PDF
- 20.World Health Organization (WHO). Surveillance guidelines for measles, rubella and congenital rubella syndrome in WHO European Region. WHO Regional Office for Europe; December 2012. Available at: http://www.euro.who.int/__data/assets/pdf_file/0018/79020/e93035-2013.pdf [PubMed]
- 21.Ministero della Salute. Italy (1998). Aggiornamento del d.m. 6 marzo 1995 concernente l'aggiornamento del d.m. 14 aprile 1984 recante protocolli di accesso agli esami di laboratorio e di diagnostica strumentale per le donne in stato di gravidanza ed a tutela della maternità. G.U. Serie Generale, n. 245 del 20 ottobre 1998. Italian. Available at: http://www.salute.gov.it/imgs/C_17_normativa_1653_allegato.pdf
- 22.Ministero della Salute. Italy (2012). Piano della Prevenzione Vaccinale 2012-2014. Intesa Stato-Regioni del 22 febbraio 2012. G.U. Serie Generale, n. 60 del 12 marzo 2012. Italian. Available at: http://www.salute.gov.it/imgs/C_17_pubblicazioni_1721_allegato.pdf
- 23.World Health Organization (WHO). Eliminating measles and rubella. Framework for the verification process in the WHO European Region. WHO Regional Office for Europe, 2014. Available at: http://www.euro.who.int/__data/assets/pdf_file/0009/247356/Eliminating-measles-and-rubella-Framework-for-the-verification-process-in-the-WHO-European-Region.pdf?ua=1
- 24.Ministero della Salute. Italy (1992). La compilazione, la codifica e la gestione della scheda di dimissione ospedaliera istituita ex DM 28/12/1991. Italian. Available at: http://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=0&codLeg=25042&parte=1%20&serie