Abstract
Highly pathogenic avian influenza H5N1 infection remains a public health threat and vaccination is the best measure of limiting the impact of a potential pandemic. Mucosal vaccines have the advantage of eliciting immune responses at the site of viral entry, thereby preventing infection as well as further viral transmission. In this study, we assessed the protective efficacy of hemagglutinin (HA) from the A/Indonesia/05/05 (H5N1) strain of influenza virus that was produced by transient expression in plants. The plant-derived vaccine, in combination with the mucosal adjuvant (3′,5′)-cyclic dimeric guanylic acid (c-di-GMP) was used for intranasal immunization of mice and ferrets, before challenge with a lethal dose of the A/Indonesia/05/05 (H5N1) virus. Mice vaccinated with 15 μg or 5 μg of adjuvanted HA survived the viral challenge, while all control mice died within 10 d of challenge. Vaccinated animals elicited serum hemagglutination inhibition, IgG and IgA antibody titers. In the ferret challenge study, all animals vaccinated with the adjuvanted plant vaccine survived the lethal viral challenge, while 50% of the control animals died. In both the mouse and ferret models, the vaccinated animals were better protected from weight loss and body temperature changes associated with H5N1 infection compared with the non-vaccinated controls. Furthermore, the systemic spread of the virus was lower in the vaccinated animals compared with the controls. Results presented here suggest that the plant-produced HA-based influenza vaccine adjuvanted with c-di-GMP is a promising vaccine/adjuvant combination for the development of new mucosal influenza vaccines.
Keywords: adjuvant; c-di-GMP; influenza H5N1; intranasal vaccination; ferret infection model; mice, plant vaccine
Abbreviations
- c-di-GMP
(3′, 5′)-cyclic dimeric guanylic acid
Introduction
The highly pathogenic influenza (HPAI) virus subtype H5N1 continues to be a serious public health risk. Human cases have recently been reported in Cambodia, Vietnam, Egypt, Indonesia and elsewhere with a case fatality rate exceeding 60%.1 HPAI viruses have not acquired transmissibility in humans, however reassortant viruses containing HA from avian H5N1 and gene segments from human H1N1 strains have been shown to efficiently transmit in ferrets2 and guinea pigs3 by exchange of respiratory droplets. Furthermore, Herfst et al.4 reported that the A/Indonesia/05/05 (H5N1) virus can acquire mutations during passage and become transmissible by aerosol or respiratory droplets in ferrets. Humans do not have pre-existing immunity to influenza viruses with H5 HA, therefore if a HPAI virus acquires transmissibility in humans, it would most likely cause a pandemic.
Vaccination is the best available measure of limiting the impact of an influenza pandemic. Ideally, a candidate pandemic influenza vaccine should be easy to administer, elicit strong mucosal and systemic immune responses, and provide broad, long-lasting immunity. Transient plant expression systems are a rapid, cost effective and easily scalable new strategy for influenza vaccine production to overcome the bottleneck in vaccine supply during a pandemic.5 This is especially important in the event of an influenza pandemic of an avian origin, as the HPAI virus kills chick embryos and hens’ eggs that are used to produce most commercial influenza vaccines. Furthermore, a needle-free intranasal (IN) influenza vaccine is an attractive approach providing immunity at the portal of virus entry and overcoming the problems of limited healthcare providers. An earlier study reported that IN vaccination with an Escherichia coli heat-labile toxin (LT) adjuvanted influenza virosomes increase the risk of Bell's palsy.6 However it was later discovered that this association was probably due to the adjuvant, as another LT-adjuvanted IN vaccine (not influenza) was also associated with Bell's palsy.7
The bacterial c-di-GMP is an intracellular signaling molecule that acts as a danger signal for eukaryotic cells (for a review see ref.8) and several studies have identified its potential as an adjuvant for mucosal and systemic vaccination.9-12 We have previously demonstrated immunostimulatory properties of c-di-GMP when used as a mucosal influenza vaccine adjuvant in mice.11,12 IN or sublingual administration of c-di-GMP adjuvanted influenza vaccines was shown to induce strong homologous and cross reactive mucosal and systemic humoral immune responses, with a balanced Th1/Th2 profile and high frequencies of multifunctional Th1 CD4+ T cells producing interferon-γ (IFN- γ), tumor necrosis factor-α (TNF- α) and interleukin-2 (IL-2).
The current study investigated the ability of a c-di-GMP adjuvanted, plant-derived H5 HA antigen to protect against a lethal H5N1 influenza virus challenge in the murine and ferret models of infection. We found that the IN administered c-di-GMP adjuvanted vaccine provided protective immunity in both mice and ferrets, therefore it is a promising mucosal vaccine formulation for pre-pandemic influenza vaccine development.
Results
Immunogenicity and protective efficacy of H5 vaccine in mice
Mouse immunogenicity study
To assess the immunogenicity of the plant-derived H5 HA vaccine adjuvanted with c-di-GMP, mice were immunized IN in a prime boost regimen with 15, 5 or 1.5 μg of HA formulated with 5 μg cd-i-GMP or with 5 μg cd-i-GMP/PBS (control). Post-vaccination serum IgG responses were examined by ELISA. In all 3 HA-vaccinated groups, the IgG levels at day 21 were comparable to pre-vaccination levels (day 0), but were significantly greater (P < 0.05) following the 2nd vaccine dose (days 28, 35 and 42; Fig. 1A). No increase in the serum IgG response was observed in control mice on any of the days tested. The serum IgA response was examined pre vaccination and 35 d post vaccination (Fig. 1B). In all 3 HA-vaccinated groups, the IgA response was significantly higher at day 35 compared with the pre-vaccination levels (day 0), but no increase was detected in the control mice. The IgA response was not significantly different at day 35 between groups that received the 15, 5 or 1.5 μg HA vaccine dose.
Figure 1.
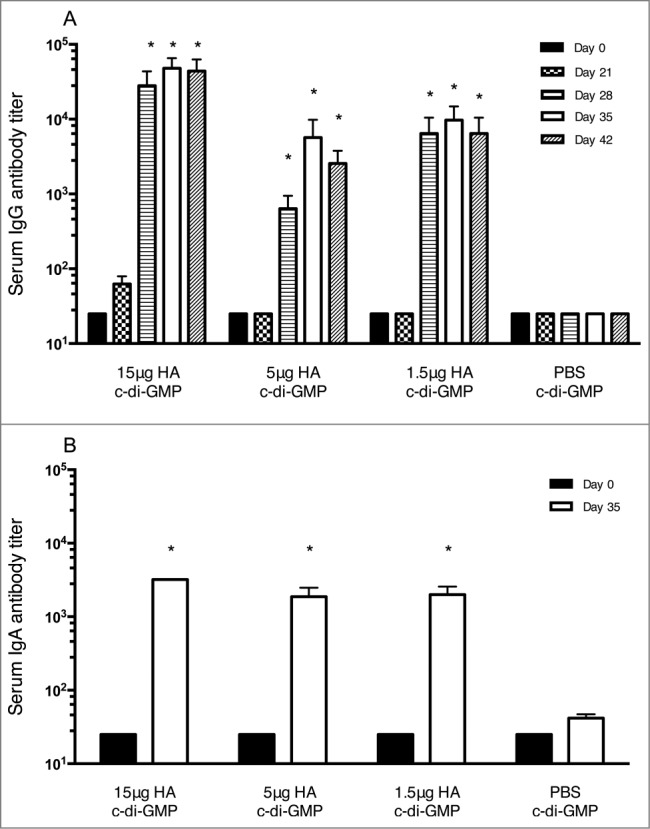
HA-specific serum IgG (A) and IgA (B) responses in mice in the immunogenicity study. Groups of 6 mice were immunized twice (21 day interval) intranasally with plant derived H1N1 vaccine at 15, 5 or 1.5 μg HA in combination with 5 μg of c-di-GMP or with PBS plus 5 μg cdi-GMP (control). Blood samples were collected at days 0, 21, 28, 35 and 42 and serum IgG and IgA levels were analyzed by ELISA. * = significantly (P < 0.05) different from Day 0 titers.
Murine challenge study
To evaluate the protective efficacy of the vaccine, mice received 2 IN doses (21 d apart) of vaccine at 15, 5 or 1.5 μg HA formulated with 5 μg cdi-GMP or with 5 μg cdi-GMP/PBS (controls) and were challenged with HPAI A/Indonesia/05/05 (H5N1) on study day 42. HI assays were conducted with serum collected pre-vaccination (Day −1) and at days 20, 32 and 41 post-vaccination (1 day before virus challenge, Fig. 2). No HI response was detected pre-vaccination or after the first immunization (day 20). After the second dose (days 32 and 41), the highest HI titers were observed in the 15 μg HA group, with lower HI titers detected in the 5 and 1.5 μg HA groups. An HI titer of ≥40 is considered protective against seasonal influenza strains in man.13 As shown in Figure 2, at 32 and 41 days post-vaccination, 7 and 5 mice, respectively from the 15 μg HA vaccine group had HI titers ≥40. In the 5 μg HA vaccine group, 6 mice had HI titers ≥40 at 32 days after vaccination. No animals in the 1.5 μg HA vaccine or in the control group had HI titers ≥40 after the second vaccine dose.
Figure 2.
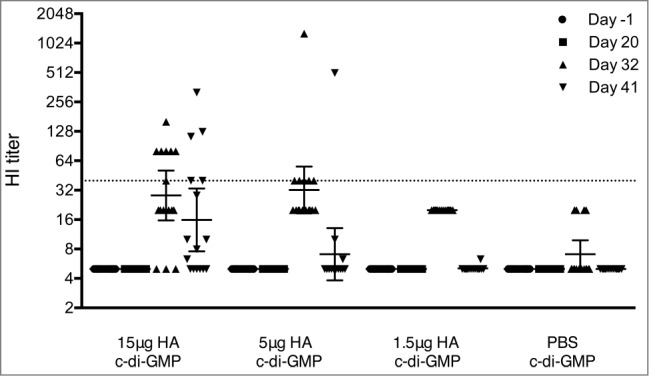
Serum HI antibody response in mice before challenge with the A/Indonesia/05/2005 (H5N1) virus. Groups of 16 mice were immunized twice (21 day interval) intranasally with plant derived vaccine at 15, 5 or 1.5 μg HA in combination with 5 μg of c-di-GMP or with PBS plus 5 μg cdi-GMP (control). Blood samples were collected pre-vaccination (Day −1) and at 20, 32, and 41 days post vaccination. The data show HI responses of each individual mouse and the horizontal lines represent geometric mean titers ± 95% confidence interval. The dotted line represents an HI titer of 40.
After viral challenge, 3 mice from each group were selected at days 3 (day 45) and 5 (day 47) post-challenge to test for the presence of virus in the upper (nasal turbinates, Fig. 3A) and lower (lung tissue, Fig. 3B) respiratory tract. Viral loads in the nasal turbinates collected on both days were at or slightly above the detection limit of 102.5 tissue culture infectious dose (TCID)50/mL and were comparable across the 3 HA-vaccinated groups and the control animals. The limit of detection for all lung tissues was 101.5 TCID50/mL. The highest viral loads were detected in the control group on both days 45 and 47. Three days post viral challenge (day 45), mice vaccinated with 15 μg or 5 μg of HA had no detectable virus in the lungs. In contrast, mice vaccinated with 1.5 μg HA and control groups were positive for viral load with group mean titers of 105 and 107.3 TCID50/mL, respectively. Five days post challenge (day 47), a dose response was observed with the group mean titers decreasing with an increasing vaccine dose with titers of 102.9, 103.8, 104.4 and 105.5 TCID50/mL for 15, 5, 1.5 μg HA and control groups, respectively.
Figure 3.
Virus titers in the upper (nasal turbinates, A) and lower (lungs, B) respiratory tract after challenge with the A/Indonesia/05/2005 (H5N1) virus. Groups of 16 mice were immunized twice (21 day interval) intranasally with plant derived vaccine at 15, 5 or 1.5 μg HA in combination with 5 μg c-di-GMP or with 5 μg c-di-GMP/PBS (control). Mice were challenged by intranasal administration of highly pathogenic avian Influenza A/Indonesia/05/2005 (H5N1) on day 42. Groups of 3 mice were euthanized at days 3 (Day 45) and 5 (Day 47) post-challenge to examine for the presence of virus in the upper (nasal turbinates, A) and lower (lungs, B) respiratory tract. The limit of detection was 102.5 TCID50/mL for nasal turbinates and 101.5 TCID50/mL for lung tissue.
All animals were monitored for body weight and clinical signs of disease on days 0, 7, 14, 21, 28, 35, 38 and 42 pre-challenge (data not shown) and daily post-challenge for 2 weeks (Fig. 4). The mean body weights in the 15 μg vaccine group remained stable until the scheduled euthanasia on day 14 post-challenge (Fig. 4A). Mice vaccinated with 5 μg HA showed a temporary loss of body weight between days 3 to 5 before they regained weight between days 6 and 14 post-challenge (Fig. 4A). All animals vaccinated with either 15 or 5 μg HA survived viral challenge to the scheduled euthanasia on day 14 (Fig. 4B). Mice vaccinated with 1.5 μg of HA lost weight between days 2 to 6 and then regained weight with minor fluctuations between days 7 and 14, however, only 80% of animals in this low dose group survived the virus challenge. In the control group, the mean body weight decreased from day 3 through to day 9, when all animals either died or were euthanized in moribund condition due to signs of severe clinical infection and/or significant weight loss (Fig. 4B).
Figure 4.
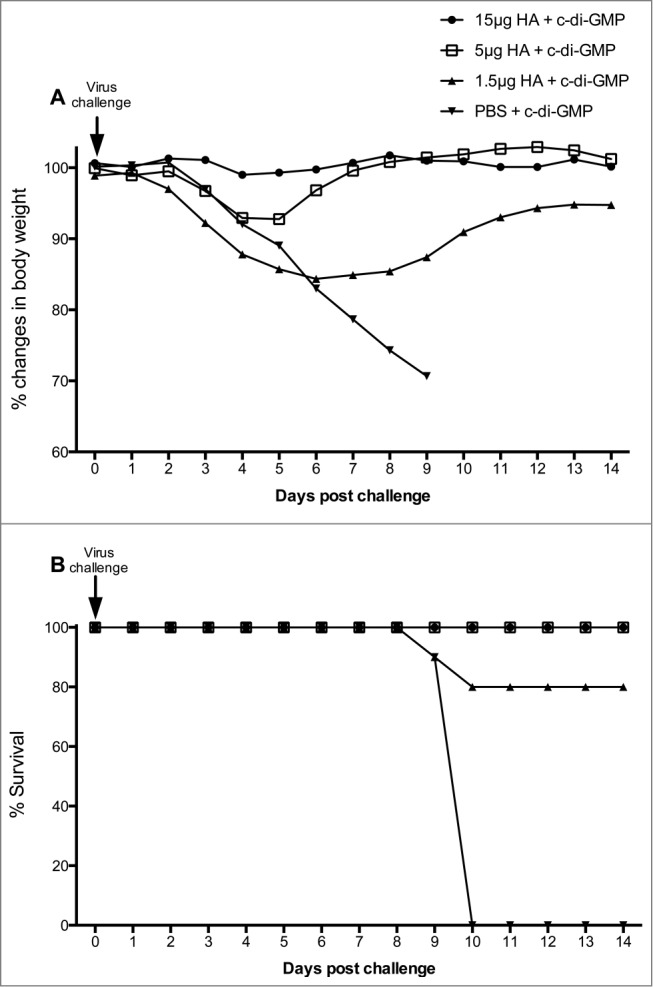
Changes in body weight (A) and mortality (B) of vaccinated mice after the A/Indonesia/05/2005 (H5N1) virus challenge. Groups of 16 mice were immunized twice (21 day interval) intranasally with the adjuvanted vaccine at a HA content of 15, 5 or 1.5 μg or with 5 μg c-di-GMP/PBS (control). Mice were challenged by intranasal administration of highly pathogenic avian Influenza A/Indonesia/05/2005 (H5N1) on day 42. (A); Body weights were obtained daily for 2 weeks post-challenge (days 0-14). The weight of the animals as measured on the day of viral challenge (day 0) was used as the baseline weight to determine changes in body weight post viral challenge. (B); The survival rate (percentage) of mice was examined daily for 2 weeks post virus challenge.
Protective efficacy of H5 vaccine in ferrets
The HA/c-di-GMP vaccine was further evaluated in a ferret model of influenza infection. In this study, ferrets were vaccinated IN twice 14 day apart with 45 μg HA vaccine with or without 50 μg c-di-GMP or with PBS alone (control), mimicking a shorter vaccination schedule, which could be appropriate in a pandemic scenario.
Serum samples were collected 12 days after each vaccination and assayed for HI antibody against A/Indonesia/05/2005 using horse erythrocytes. No serum antibody responses were detected after the first or the second vaccination in any of the ferrets (results not shown).
The ferrets were challenged IN 14 days after the second vaccine dose with A/Indonesia/05/2005 live virus. Three days post-viral challenge, 5 ferrets per group were sacrificed and lung, nasal turbinate, brain, olfactory bulb, spleen and nasal wash samples were collected. The mean titers of virus recovered from the control animals were higher compared to the vaccinated animals for all tissues, although the differences were not statistically significant (Fig. 5). Lower viral titers were recovered in the nasal turbinates from the animals given the adjuvanted vaccine than in the animals that had received HA alone or PBS. For both the vaccinated and control ferrets, lower virus titers were recovered systemically (brain, olfactory bulb and the spleen) than in the respiratory tract recovered from lungs and nasal turbinates.
Figure 5.
Virus recovery from the respiratory tract and systemic organs of vaccinated ferrets after challenge with A/Indonesia/05/2005 (H5N1) virus. Ferrets were vaccinated with 2 doses of the plant-derived vaccine (45 μg HA) adjuvanted with c-di-GMP (50 μg), non-adjuvanted vaccine or PBS (control) 14 days apart by the intranasal route. The ferrets were then challenged at 14 days after the second vaccine dose with the A/Indonesia/05/2005 (H5N1) live virus by the intranasal route. On day 3 post-challenge, 5 ferrets from each group were sacrificed and lung, nasal turbinate (NT), brain, olfactory bulb, spleen and nasal wash samples were collected. Tissue samples were homogenized and the clarified homogenates and nasal washes were assayed for the presence of A/Indonesia/05/2005 virus by titration on MDCK cells.
The survival rates of ferrets, excluding animals sacrificed on day 3 post-challenge are shown in Table 1. All ferrets vaccinated with the c-di-GMP adjuvanted vaccine survived the viral challenge, whereas 2 out of 7 animals vaccinated with the non-adjuvanted vaccine succumbed to infection. In the control group, 4 out of 8 ferrets survived. The difference in survival rates between the control and the adjuvanted vaccine groups was marginal (p = 0.077).
Table 1.
Survival rates, temperature rise and weight loss in vaccinated and control ferrets after the A/Indonesia/05/2005 (H5N1) virus challenge. Ferrets were vaccinated with 2 doses of c-di-GMP adjuvanted plant-derived vaccine, vaccine alone and PBS (control) 14 days apart by the intranasal route. The ferrets were then challenged 14 days after the second vaccine dose with the A/Indonesia/05/2005 (H5N1) live virus by the intranasal route. On day 14 post-challenge, the surviving animals were exsanguinated under terminal anesthesia
| Vaccine | Survival* (%) | No. animals temperature rise >1°C | Mean temperature rise day 2 (°C) | Mean temperature rise day 3 (°C) | Weight loss ≥5%* | Weight loss ≥10%* |
|---|---|---|---|---|---|---|
| 45 μg HA | 5/7 (71%) | 9/12 | 0.8 | 1.3 | 4/7 | 3/7 |
| 45 μg HA + 50 μg c-di-GMP | 7/7 (100%) | 10/12 | 0.9 | 0.9 | 4/7 | 2/7 |
| PBS (controls) | 4/8 (50%) | 13/13 | 1.7 | 1.6 | 8/8 | 6/8 |
* Excluding the animals sacrificed on day 3 for post-challenge virus recovery (Fig. 5).
The body weight of the ferrets was monitored for 14 days post-viral challenge. The greatest weight loss was observed in control animals whereas ferrets given the adjuvanted vaccine showed the least amount of weight loss over the 2-week period (Fig. 6A). Of the animals that lost weight and survived, half had regained weight by day 14. Excluding the animals sacrificed on day 3, 4 out of 7 animals in each of the vaccinated groups and all of the control animals lost ≥5% weight after challenge (Table 1). The maximum mean weight loss was observed in the control group and similar weight losses were observed in the 2 vaccinated groups (Table 1). Three animals in the vaccine alone and 2 ferrets in the adjuvanted vaccine group experienced a serious weight loss (≥10%), whereas the majority of the controls (6/8) had this level of weight loss (Table 1).
Figure 6.
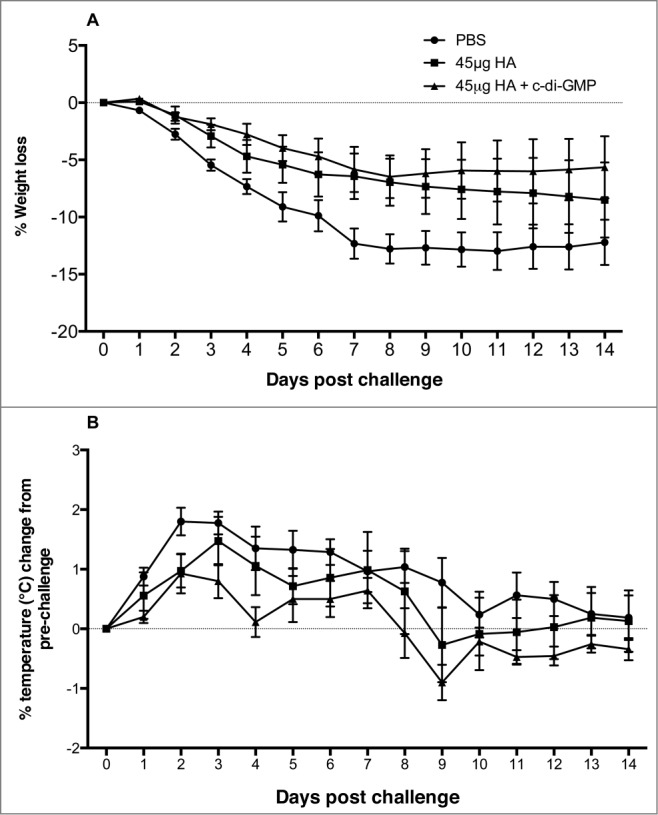
Percentage weight loss (A) and changes in body temperature (B) in vaccinated and control ferrets following challenge with the A/Indonesia/05/2005 (H5N1) virus. Ferrets were vaccinated with 2 doses of 50 μg c-di-GMP adjuvanted vaccine (45 μg HA + c-di-GMP), vaccine alone (45 μg HA) and PBS (control) 14 days apart by the intranasal route. The ferrets were then challenged 14 days after the second vaccine dose with the A/Indonesia/05/2005 (H5N1) live virus by the intranasal route. Ferrets were monitored daily for 14 days following viral challenge for body weight and temperature.
Following viral challenge of ferrets, changes in body temperature were monitored daily for 14 days (Fig. 6B). All the control animals (13/13) and 9/12 and 10/12 vaccinated animals in the vaccine alone or adjuvanted vaccine groups, respectively, developed fever (temperature rise >1°C) following viral challenge (Table 1). The maximum mean temperature rises were recorded at 2 or 3 days post vaccination. The temperature remained stable in the control and adjuvanted vaccine animals between days 2 and 3 post-challenge, whereas an increase in temperature was recorded in the vaccine alone group from 0.8 to 1.3°C.
Discussion
Since 2003, over 700 confirmed human cases of avian influenza A(H5N1) have been reported to the World Health Organization with a case fatality rate of approximately 60%.1 With its high virulence, avian influenza viruses pose a threat to global public health, but a lack of sustained transmission between humans has prevented more widespread outbreaks. However, recent studies have discovered determinants of sustained airborne transmission of H5N1 viruses in the ferret model.2,4 Ferrets are a natural host of influenza and represent a well-established model for influenza research (for a review see ref.14), although it is not clear whether mutations that led to transmissibility in ferrets will mediate sustained human-to-human transmission.2 However, it is now clear that H5N1 viruses can transmit between mammals and may continue to evolve becoming transmissible in humans, resulting in a pandemic in the global naïve population.
The 2009 H1N1 pandemic clearly demonstrated that we do not have the capacity to produce enough vaccine doses in a timely manner to meet the global requirements during a pandemic. In addition, in the event of an avian influenza pandemic, traditional use of embryonated hens’ eggs for vaccine production is not a viable option.15 Therefore, the development of new manufacturing platforms, novel adjuvants and dose-sparing strategies is a priority. Some of the alternative influenza vaccines being explored include DNA vaccines, reverse genetics engineered vaccines and cell-based vaccines using either Vero or MDCK cells.16 We have previously described a transient plant-based expression system for producing HA antigens as a low cost and time efficient alternative to more traditional vaccine manufacturing methods.17 As with most candidate H5N1 mucosal vaccines, the plant-derived vaccines require an effective adjuvant to enhance the immunogenicity and maximise the number of doses obtained from each production batch.5,18 Here, IN administration of a plant-derived H5 vaccine in combination with the novel adjuvant c-di-GMP showed great promise in the mouse and ferret models, protecting animals from morbidity and mortality associated with the highly pathogenic avian influenza virus challenge. Furthermore, vaccination reduced febrile responses, severe weight loss and prevented the systemic spread of the virus. The immunogenicity results described in this study for the plant-derived vaccine are comparable to those reported by Shoji et al.,19 although relatively higher antigen doses (90 and 45 μg HA) and a different adjuvant (Quil A) were used in their study and importantly, the animals were immunized subcutaneously. To our knowledge, the current study is the first showing protective efficacy of a c-di-GMP-adjuvanted, plant-derived influenza vaccine in mice and ferrets after mucosal (IN) vaccination. The development of a vaccine/adjuvant formulation that elicits effective mucosal immune responses will be beneficial in preventing infection and replication at the site of influenza virus entry (respiratory tract). Furthermore, a mucosal vaccine may reduce viral shedding and horizontal transmission to susceptible hosts. In addition, the needle-free self-administration of mucosal vaccines and associated easier logistics may improve public compliance, leading to improved immunization coverage.
We have also studied the humoral responses induced by IN vaccination with the plant-derived HA/c-di-GMP influenza vaccine and found that high levels of serum IgG and IgA were induced in mice after 2 vaccine doses. The increased serum IgA response may indicate the mucosal origin of these antibodies and activation of mucosal IgA B lymphocytes.20 In addition, serum HI antibody was observed in mice after immunization with high vaccine doses (15 μg and 5 μg of HA). In contrast, IN vaccination failed to induce a serum HI antibody response in ferrets. These results differ from those previous reported by Shoji et al.19 where subcutaneous vaccination with a high dose of a plant-derived influenza vaccine (90 μg HA, Quil A adjuvanted) induced a strong serum HI antibody response. Therefore, it will be interesting to study whether the route of administration or the adjuvant used determined the serum HI antibody response in ferrets. Despite the lack of serum HI antibody responses, all ferrets in the adjuvanted vaccine group and 80% of mice that received the 1.5 μg HA vaccine survived the lethal virus challenge. In fact, the lack of correlation between circulating HI antibody titers and protective efficacy has been noted previously in ferrets immunized with pandemic avian influenza vaccines.21,22 Low serum antibody responses are also a major concern in human clinical trials of pandemic influenza vaccines (for a review see ref.23). However, low HI antibody titers may not reflect the true protective potential of these vaccines as other factors, especially priming of a cellular immune response and associated cytokine production, may play a significant role in providing clinical protection. We have recently shown that IN administration of the plant-derived HA/cd-i-GMP vaccine induced strong humoral and cellular immune responses, including a balanced Th1/Th2 cytokine and antibody responses and high frequencies of multifunctional Th1 CD4+ T cells.11 Taken together, our findings suggest that an effective cellular response may at least in part explain the high levels of protection observed in ferrets despite the low titers of serum HI antibodies.
Several pre-clinical studies have convincingly shown the potent immunostimulatory properties of c-di-GMP when used as a mucosal adjuvant (for a review see ref.8). The mechanism by which c-di-GMP stimulates a host immune response is now being investigated and the transmembrane protein stimulator of interferon genes (STING) has been shown to function as a sensor for c-di-GMP and other cyclic dinucleotides (CDNs).24 A proposed mechanism for c-di-GMPs adjuvant properties is that STING ligation increases the production of type I interferons,25 which in turn drives the adaptive immune response. Parvatiyar et al.26 showed that cellular DEAD (aspartate-glutamate-alanine-aspartate)-box helicase DDX41 operates ‘upstream’ of STING to sense c-di-GMP and other CDNs and is responsible for activating the STING-dependent pathway that activates the interferon response. Interestingly, recent studies suggested that STING-dependent, but IRF3-independent, stimulation of NF-κB signaling leading to TNF-α production is critical for the adjuvant activity of c-di-GMP.27
The protective efficacy of the plant derived H5 vaccine was further examined by evaluating the viral load in the respiratory tract and systemic organs following immunization and virus challenge. In both ferrets and humans, highly pathogenic H5N1 viruses often target the lower respiratory tract (LRT), especially lungs, mainly due to the presence of α2-3-linked sialic acid receptors and high viral loads in the LRT are often associated with clinical signs of severe H5N1 infections.28 In our mouse model, the reduced viral load in the lung tissue samples on days 3 and 5 post-challenge indicate that administration of the adjuvanted vaccine may have reduced the spread of the virus to the lungs. These findings were confirmed in the larger ferret model, where administration of the adjuvanted vaccine reduced the viral load in the respiratory tract, and importantly, limited systemic virus spread to the olfactory bulb, brain and spleen, which may have played a significant role in protecting the animals from the lethal H5N1 virus challenge.29 Earlier studies have reported a strong epidemiological association between IN vaccination and facial nerve paralysis (Bell's palsy) and this was attributed to the use of E. coli LT as an adjuvant.6,7 However, there is no evidence to suggest that IN vaccination with c-di-GMP can cause Bell's palsy.
In conclusion, we have shown here for the first time that IN administration of a plant-derived influenza vaccine provide protection against highly pathogenic avian influenza virus challenge, making it a promising candidate for a future development as an intransal influenza vaccine. The protective response was achieved despite low antibody titers, suggesting that HI titers alone are not a correlate of protection for a H5 vaccine candidate and that further pre-clinical and human studies are needed to determine these correlates.
Materials and Methods
Virus strains and vaccine preparation
Virus strain
The highly pathogenic A/Indonesia/05/2005 (H5N1) virus was received from the Centers for Disease Control and Prevention (Atlanta, GA, USA) or Queen Mary Hospital, Hong Kong and propagated in embryonated hens’ eggs. The allantoic fluid was aliquoted and stored at −70°C for use in challenge experiments.
Vaccine preparation
The recombinant vaccine was expressed in tobacco plants by agrobacterium gene transfer as described previously.19,30 Briefly, amino acids 17-532 of the HA gene of the A/Indonesia/05/2005 (H5N1) virus were cloned into a plasmid vector. This vector, together with a helper plasmid, was transformed into Agrobacterium tumefaciens. The transformed bacteria were cultured overnight and subsequently vacuum infiltrated into 6-week-old Nicotiana benthamiana plants. After seven days, leaves were harvested and homogenized, the extracts were clarified by centrifugation, and the HA protein was purified by immobilized metal affinity chromatography and anion exchange chromatography.
Adjuvant preparation
The c-di-GMP adjuvant was produced as previously described at Helmholtz Center for Infection Research, Germany.9,31 The plant-derived vaccine was mixed with the adjuvant immediately prior to vaccination.
Immunogenicity and protective efficacy in mice
The mouse immunogenicity study was conducted by Fraunhofer USA Center for Molecular Biotechnology (FhCMB) at the University of Delaware's Office of Laboratory Animal Medicine (Newark, DE, USA). The animal use protocol was approved by the University of Delaware Institutional Animal Care and Use Committee (IACUC). The mouse challenge study was conducted at Southern Research Institute's laboratories in Birmingham, AL, USA that are fully accredited by the Associate for the Assessment and Accreditation of Laboratory Animal Care, International (AAALAC). Approval for these studies was given by the Institutional Animal Care and Use Committee (IACUC) at Southern Research Institute.
Immunogenicity in mice
Groups of 6-week-old BALB/c mice (Harlan Laboratories, Indianapolis, IN), 6 mice per group, were immunized IN with plant-derived vaccine in the presence or absence of 5 μg of c-di-GMP at 3-week interval on study days 0 and 21. Three different doses of the vaccine were tested: 15, 5, and 1.5 μg HA. Animals in the negative control group received PBS plus 5 μg of c-di-GMP. Serum samples were collected prior to each immunization and 1, 2, and 3 weeks after the last immunization corresponding to study days 28, 35, and 42, respectively. Serum IgG, IgA antibody titers were determined.
Viral challenge study in mice
BALB/c mice (7-11 week old females from Charles River Laboratories, Kingston, NY) were randomized into 4 groups (16/mice per group). For IN administration of the vaccine, mice were anesthetized with Isoflurane to effect and administered 10 μL containing 15 μg, 5 μg, or 1.5 μg of HA in combination with 5 μg of cdi-GMP IN on study days 0 and 21. Control mice received 5 μg of cdi-GMP plus PBS on days 0 and 21. On day 42 all mice were anesthetized by intraperitoneal administration of Ketamine/Xylazine (50 mg/kg Ketamine and 5 mg/kg Xylazine), then challenged IN with A/Indonesia/05/2005 (H5N1) virus (90 μl, added in a drop wise manner, 102.23 EID50/mL). A Bair Hugger warm air unit was used to keep animals warm during recovery from anesthesia. Body temperatures and weight were recorded on days 0, 7, 14, 21, 28, 35, 38 and 42 pre-challenge, then daily post-challenge from day 43 to 56. For pre-vaccination blood collection (day −1), mice were anesthetized with CO2/O2 inhalation and approximately 100 μl of blood from each mouse was collected from the retro-orbital sinus. On days 20, 32, and 41, approximately 100 μl of blood was collected via the submandibular route from each non-anesthetized mouse and serum stored at −70°C. On days 45 and 47 (3 and 5 days post challenge), 3 mice from each group were sacrificed by CO2 inhalation. The nasal turbinates and lungs were collected, snap frozen (dry ice) and stored (−80 ± 10°C) until evaluation for viral load in Mardin Darby canine kidney (MDCK) cells. All remaining animals were sacrificed on day 56 by CO2 inhalation.
Vaccination and viral challenge of ferrets
All procedures in the ferret challenge study were carried out according the UK Home Office License regulations and by individuals with appropriate personal Home Office Licenses. The study was approved by the local ethics committee.
Adult male ferrets (Mustela putorius furo, accredited supplier, UK) at 7-9 months of age were randomly assigned to one of 3 study groups. Ferrets were housed in open pens after vaccination and then transferred to individual cages prior to challenge. Animals were fed twice daily with ferret breeding and maintenance diet. All ferrets were seronegative as determined by HI assays (HI titers < 10) to the H5N1 virus and currently circulating H3, H1 and B influenza strains.
Animals were sedated with 0.2 mL/kg of Ketamine/Xylazine before IN immunisation with 2 doses of vaccine 14 days apart containing 45 μg of HA in PBS alone (n = 12) or with 50 μg cdi-GMP (n = 12). Control animals received PBS alone (n = 13). Animals were bled from the jugular vein pre vaccination and 12 days after each immunisation and at the time of sacrifice. Serum and samples were stored at −70°C until testing in the serological assays.
Fourteen days after the second immunisation, animals were sedated with 0.2 mL/kg of Ketamine/Xylazine and challenged by administering A/Indonesia/05/2005 live virus (106 EID50 in 0.4 mL PBS/BSA) by the IN route. From four days prior to challenge through 14 days post-challenge, all animals were monitored for fever, weight change and signs of clinical disease starting. Three days post-challenge, 5 animals from each group were sacrificed and the lung, nasal turbinate, brain, olfactory bulb, spleen and nasal washes were collected. The remaining animals were observed for clinical signs of disease until 14 days post challenge, when surviving animals were exsanguinated under terminal anesthesia. Animals whose condition exceeded the moderate severity limit of the study were exsanguinated under terminal anesthesia. All the challenge studies were conducted under enhanced BSL-3 conditions.
Virus recovery from nasal washings and tissue samples from mice and ferrets
In the mouse study, lung and nasal turbinate tissues collected on days 45 and 47 (3 and 5 days post challenge) were thawed and washed with 1% penicillin-streptomycin in PBS. Lung tissues were homogenized 1:10 (w:v) and nasal turbinates were diluted at 1:100 (w:v) in homogenization solution (DMEM with 1% penicillin and streptomycin, and 1% L-glutamine) and analyzed for replication in MDCK cells. Samples were placed on wet ice or refrigerated for up to 8 hours prior to analysis.
Immediately after collection, ferret tissues were weighed and homogenized in L15 medium containing penicillin (1000 IU) and streptomycin (100 μg/ml). Homogenates were clarified by centrifugation and aliquots frozen at −70°C. Nasal washings, and clarified tissue homogenates were inoculated undiluted or diluted (10−1–10−8) into MDCK cells in 96 well tissue culture plates and plates were incubated at 35°C for 3 days. Aliquots of medium from each well were transferred to wells of U-well microtiter plates and the presence of replicating virus detected using 0.7% turkey erythrocytes. Virus titers were calculated by the method of Spearman-Karber.32 The limit of detection for the mouse assay was 102.5 TCID50/mL for nasal turbinates and 101.5 TCID50/mL for lung tissue samples. The limit of detection for the ferret samples was 101.3/g or 101.56/mL.
Enzyme-linked immunosorbent assay (ELISA)
The influenza-specific murine serum IgG and IgA antibodies were quantified by ELISA assay, as previously described.33,34 Briefly ELISA plates were coated with inactivated A/Indonesia/05/2005 virus and samples of serum were tested in a series of 2- or 4-fold dilutions. Antigen-specific IgG and IgA were detected using horseradish peroxidase-conjugated goat anti-mouse IgG or IgA antibody (Jackson Immunoresearch Laboratories Inc., West Grove, PA, USA), respectively. Endpoint titers were determined as reciprocal serum dilutions that gave mean optical density values 3 times greater than those from pre-immune sera at a 1:100 or 1:50 dilution for IgG and IgA, respectively.
HI assay
Sera were treated to remove non-specific inhibitors by 1/4 dilution in receptor destroying enzyme (Seiken, Japan) and incubation for 18 hours at 37°C followed by 45 minute incubation at 56°C and tested by a modified HI assay.35,36 The HI assay was performed by a standard micro-titer method using 2-fold serial dilution of sera in 25 μl volumes, 4 haemagglutinating units of IBCDC-RG2 and 1% horse erythrocytes. Each serum sample was tested at least twice and the results reported as the geometric mean of the readings. The serum HI titer was expressed as the reciprocal of the highest dilution at which haemagglutination was inhibited and titers less than 10 were assigned an arbitrary value of 5 for calculation purposes.
Statistical analyses
Non-parametric Kruskall-Wallis multiple comparisons test was used to analyze differences between the groups using GraphPad Prism version 6 for Mac (GraphPad Software, La Jolla, CA, USA) and a P value < 0.05 was considered to be statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The work was supported by the Ministry of Health and Care Services, Norway, EU FP7 UniVax (601738), EU IMI115672, FLUCOP, Helse Vest, RCN Globvac (220670) and the K.G. Jebsen Centre for Influenza Vaccine Research.
References
- 1. World Health Organization Summary of assessment as of January 2015. Available from: http://www.who.int/influenza/human_animal_interface/Influenza_Summary_IRA_HA_interface_26January2015.pdf.pdf?ua=1. [Google Scholar]
- 2. Imai M, Watanabe T, Hatta M, Das SC, Ozawa M, Shinya K, Zhong G, Hanson A, Katsura H, Watanabe S, et al. . Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 2012; 486:420-8; PMID:22722205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang Y, Zhang Q, Kong H, Jiang Y, Gao Y, Deng G, Shi J, Tian G, Liu L, Liu J, et al. . H5N1 hybrid viruses bearing 2009/H1N1 virus genes transmit in guinea pigs by respiratory droplet. Science 2013; 340:1459-63; PMID:23641061; http://dx.doi.org/ 10.1126/science.1229455 [DOI] [PubMed] [Google Scholar]
- 4. Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, Sorrell EM, Bestebroer TM, Burke DF, Smith DJ, et al. . Airborne transmission of influenza A/H5N1 virus between ferrets. Science 2012; 336:1534-41; PMID:22723413; http://dx.doi.org/ 10.1126/science.1213362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chichester JA, Haaheim LR, Yusibov V. Using plant cells as influenza vaccine substrates. Expert Rev Vaccin 2009; 8:493-8; PMID:19348564; http://dx.doi.org/ 10.1586/erv.09.3 [DOI] [PubMed] [Google Scholar]
- 6. Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, Spyr C, Steffen R. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N Engl J Med 2004; 350:896-903; PMID:14985487; http://dx.doi.org/ 10.1056/NEJMoa030595 [DOI] [PubMed] [Google Scholar]
- 7. Lewis DJ, Huo Z, Barnett S, Kromann I, Giemza R, Galiza E, Woodrow M, Thierry-Carstensen B, Andersen P, Novicki D, et al. . Transient facial nerve paralysis (Bell's palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PloS One 2009; 4:e6999; PMID:19756141; http://dx.doi.org/ 10.1371/journal.pone.0006999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen W, Kuolee R, Yan H. The potential of 3′,5′-cyclic diguanylic acid (c-di-GMP) as an effective vaccine adjuvant. Vaccine 2010; 28:3080-5; PMID:20197136; http://dx.doi.org/ 10.1016/j.vaccine.2010.02.081 [DOI] [PubMed] [Google Scholar]
- 9. Ebensen T, Schulze K, Riese P, Morr M, Guzman CA. The bacterial second messenger cdiGMP exhibits promising activity as a mucosal adjuvant. Clin Vaccine Immunol 2007; 14:952-8; PMID:17567766; http://dx.doi.org/ 10.1128/CVI.00119-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karaolis DK, Means TK, Yang D, Takahashi M, Yoshimura T, Muraille E, Philpott D, Schroeder JT, Hyodo M, Hayakawa Y, et al. . Bacterial c-di-GMP is an immunostimulatory molecule. J Immunol 2007; 178:2171-81; http://dx.doi.org/ 10.4049/jimmunol.178.4.2171 [DOI] [PubMed] [Google Scholar]
- 11. Madhun AS, Haaheim LR, Nostbakken JK, Ebensen T, Chichester J, Yusibov V, Guzman CA, Cox RJ. Intranasal c-di-GMP-adjuvanted plant-derived H5 influenza vaccine induces multifunctional Th1 CD4+ cells and strong mucosal and systemic antibody responses in mice. Vaccine 2011; 29:4973-82; PMID:21600260; http://dx.doi.org/ 10.1016/j.vaccine.2011.04.094 [DOI] [PubMed] [Google Scholar]
- 12. Pedersen GK, Ebensen T, Gjeraker IH, Svindland S, Bredholt G, Guzman CA, Cox RJ. Evaluation of the sublingual route for administration of influenza H5N1 virosomes in combination with the bacterial second messenger c-di-GMP. PloS One 2011; 6:e26973; PMID:22069479; http://dx.doi.org/ 10.1371/journal.pone.0026973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg 1972; 70:767-77; PMID:4509641; http://dx.doi.org/ 10.1017/S0022172400022610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Banner D, Kelvin AA. The current state of H5N1 vaccines and the use of the ferret model for influenza therapeutic and prophylactic development. J Infect Dev Ctries 2012; 6:465-9; PMID:22706187 [DOI] [PubMed] [Google Scholar]
- 15. Wood JM, Robertson JS. From lethal virus to life-saving vaccine: developing inactivated vaccines for pandemic influenza. Nat Rev Microbiol 2004; 2:842-7; PMID:15378048; http://dx.doi.org/ 10.1038/nrmicro979 [DOI] [PubMed] [Google Scholar]
- 16. Clegg CH, Rininger JA, Baldwin SL. Clinical vaccine development for H5N1 influenza. Expert Rev Vaccin 2013; 12:767-77; PMID:23885822; http://dx.doi.org/ 10.1586/14760584.2013.811178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shoji Y, Chichester JA, Jones M, Manceva SD, Damon E, Mett V, Musiychuk K, Bi H, Farrance C, Shamloul M, et al. . Plant-based rapid production of recombinant subunit hemagglutinin vaccines targeting H1N1 and H5N1 influenza. Hum Vaccin 2011; 7 Suppl:41-50; PMID:21266846; http://dx.doi.org/ 10.4161/hv.7.0.14561 [DOI] [PubMed] [Google Scholar]
- 18. Chichester JA, Yusibov V. Plants as alternative systems for production of vaccines. Hum Vaccin 2007; 3:146-8; PMID:17643065; http://dx.doi.org/ 10.4161/hv.3.4.4148 [DOI] [PubMed] [Google Scholar]
- 19. Shoji Y, Bi H, Musiychuk K, Rhee A, Horsey A, Roy G, Green B, Shamloul M, Farrance CE, Taggart B, et al. . Plant-derived hemagglutinin protects ferrets against challenge infection with the A/Indonesia/05/05 strain of avian influenza. Vaccine 2009; 27:1087-92; PMID:19100806; http://dx.doi.org/ 10.1016/j.vaccine.2008.11.108 [DOI] [PubMed] [Google Scholar]
- 20. Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine 2007; 25:5467-84; PMID:17227687; http://dx.doi.org/ 10.1016/j.vaccine.2006.12.001 [DOI] [PubMed] [Google Scholar]
- 21. Cox RJ, Major D, Hauge S, Madhun AS, Brokstad KA, Kuhne M, Smith J, Vogel FR, Zambon M, Haaheim LR, et al. . A cell-based H7N1 split influenza virion vaccine confers protection in mouse and ferret challenge models. Influenza Other Respir Viruses 2009; 3:107-17; PMID:19453487; http://dx.doi.org/ 10.1111/j.1750-2659.2009.00082.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lipatov AS, Hoffmann E, Salomon R, Yen HL, Webster RG. Cross-protectiveness and immunogenicity of influenza A/Duck/Singapore/3/97(H5) vaccines against infection with A/Vietnam/1203/04(H5N1) virus in ferrets. J Infect Dis 2006; 194:1040-3; PMID:16991078; http://dx.doi.org/ 10.1086/507709 [DOI] [PubMed] [Google Scholar]
- 23. Baz M, Luke CJ, Cheng X, Jin H, Subbarao K. H5N1 vaccines in humans. Virus Res 2013; 178(1):78-98; PMID:23726847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE. STING is a direct innate immune sensor of cyclic di-GMP. Nature 2011; 478:515-8; PMID:21947006; http://dx.doi.org/ 10.1038/nature10429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 2009; 461:788-92; PMID:19776740; http://dx.doi.org/ 10.1038/nature08476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parvatiyar K, Zhang Z, Teles RM, Ouyang S, Jiang Y, Iyer SS, Zaver SA, Schenk M, Zeng S, Zhong W, et al. . The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat Immunol 2012; 13:1155-61; PMID:23142775; http://dx.doi.org/ 10.1038/ni.2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blaauboer SM, Gabrielle VD, Jin L. MPYS/STING-mediated TNF-alpha, not type I IFN, is essential for the mucosal adjuvant activity of (3'-5')-cyclic-di-guanosine-monophosphate in vivo. J Immunol 2014; 192:492-502; http://dx.doi.org/ 10.4049/jimmunol.1301812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Riel D, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, Osterhaus AD, Kuiken T. H5N1 Virus Attachment to Lower Respiratory Tract. Science 2006; 312:399; PMID:16556800; http://dx.doi.org/ 10.1126/science.1125548 [DOI] [PubMed] [Google Scholar]
- 29. Schrauwen EJ, Herfst S, Leijten LM, van Run P, Bestebroer TM, Linster M, Bodewes R, Kreijtz JH, Rimmelzwaan GF, Osterhaus AD, et al. . The multibasic cleavage site in H5N1 virus is critical for systemic spread along the olfactory and hematogenous routes in ferrets. J Virol 2012; 86:3975-84; PMID:22278228; http://dx.doi.org/ 10.1128/JVI.06828-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shoji Y, Chichester JA, Bi H, Musiychuk K, de la Rosa P, Goldschmidt L, Horsey A, Ugulava N, Palmer GA, Mett V, et al. . Plant-expressed HA as a seasonal influenza vaccine candidate. Vaccine 2008; 26:2930-4; PMID:18440103; http://dx.doi.org/ 10.1016/j.vaccine.2008.03.045 [DOI] [PubMed] [Google Scholar]
- 31. Ebensen T, Schulze K, Riese P, Link C, Morr M, Guzman CA. The bacterial second messenger cyclic diGMP exhibits potent adjuvant properties. Vaccine 2007; 25:1464-9; PMID:17187906; http://dx.doi.org/ 10.1016/j.vaccine.2006.10.033 [DOI] [PubMed] [Google Scholar]
- 32. Mahy BWJ, Kangro HO. Virology methods manual. New York: Academic Press Limited, 1996; page 37. ISBN 0-12-465330-8. [Google Scholar]
- 33. Hauge S, Madhun A, Cox RJ, Haaheim LR. Quality and kinetics of the antibody response in mice after three different low-dose influenza virus vaccination strategies. Clin Vaccine Immunol 2007; 14:978-83; PMID:17596426; http://dx.doi.org/ 10.1128/CVI.00033-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hovden AO, Cox RJ, Haaheim LR. Whole influenza virus vaccine is more immunogenic than split influenza virus vaccine and induces primarily an IgG2a response in BALB/c mice. Scand J Immunol 2005; 62:36-44; PMID:16092921; http://dx.doi.org/ 10.1111/j.1365-3083.2005.01633.x [DOI] [PubMed] [Google Scholar]
- 35. Stephenson I, Wood JM, Nicholson KG, Charlett A, Zambon MC. Detection of anti-H5 responses in human sera by HI using horse erythrocytes following MF59-adjuvanted influenza A/Duck/Singapore/97 vaccine. Virus Res 2004; 103:91-5; PMID:15163495; http://dx.doi.org/ 10.1016/j.virusres.2004.02.019 [DOI] [PubMed] [Google Scholar]
- 36. Bresson JL, Perronne C, Launay O, Gerdil C, Saville M, Wood J, Hoschler K, Zambon MC. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet 2006; 367:1657-64; PMID:16714186; http://dx.doi.org/ 10.1016/S0140-6736(06)68656-X [DOI] [PubMed] [Google Scholar]


