Fig. 1. Cysteine replacements in CheW and Tsr.
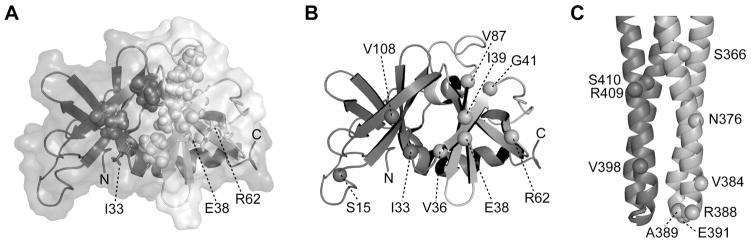
(A) CheW structure (PDB 2HO9, Li et al., 2007). Subdomains 1 and 2 are coloured in dark- and light-grey respectively. Residues in the hydrophobic groove that are postulated to interact with receptors are shown in space-fill representation. Residues I33, E38 and R62 are shown as sticks.
(B) Backbone structure of CheW. Labelled spheres indicate the residue positions chosen for cysteine reporters.
(C) The cytoplasmic hairpin tip of a Tsr dimer (PDB 1QU7, Kim et al., 1999). The spheres in the darker subunit show C-helix residues chosen for cysteine reporters; spheres in the lighter subunit show N-helix residues chosen for cysteine reporters.
