Figure 2.
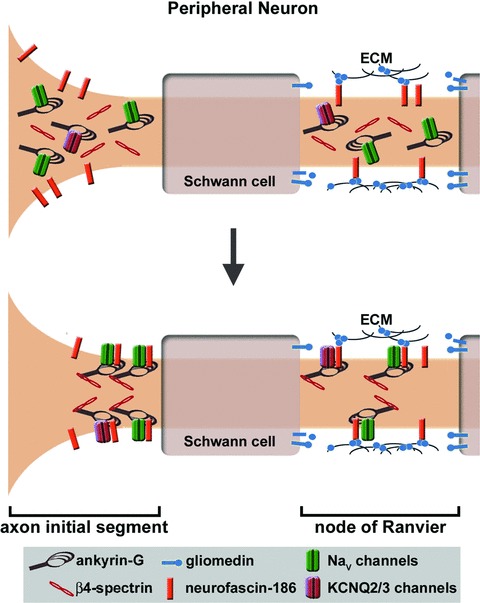
Ankyrin-G targeting to membrane domains in the peripheral neuron. Ankyrin-G is recruited to the nodes of Ranvier by gliomedin, which is produced by Schwann cells and accumulates in the perinodal extracellular matrix. As a ligand for neurofascin-186, gliomedin causes the nodal clustering of this cell adhesion molecule, which in turn recruits to the nodal plasma membrane an ankyrin-G protein network consisting of voltage-gated sodium or potassium channels (KCNQ2/3) and β4-spectrin. In contrast, ankyrin-G localization to the AIS is not dependent on an extracellular cue or neurofascin-186. The AIS targeting of ankyrin-G appears to be mediated by an intrinsic mechanism that has yet to be discovered, but AIS targeting of ankyrin-G associated proteins (i.e. neurofascin-186, sodium and potassium channels, β4-spectrin) is dependent on ankyrin-G.
