Figure 4.
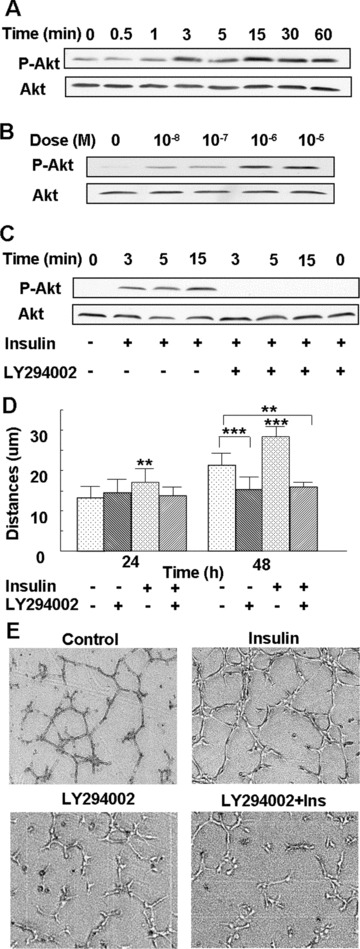
PI3K-Akt mediates insulin-induced microvascular endothelial cell migration and tube formation: (A) Endothelial cells were treated with 10−7 M insulin for the indicated time-points, followed by Western blot analysis using an antibody directed against Akt phosphorylated at Ser 473. The blot was re-probed with an anti-Akt Ab to ensure equal loading. Insulin increased Akt phosphorylation over time, with peak phosphorylation seen at 3 and 15 min. (B) Cells were either left untreated or treated with 10−8 M to10−5 M insulin for 3 min., and then analysed as in (A). Insulin stimulated phosphorylation of Akt in a dose-dependent manner, with 10−6 M and 10−5 M insulin inducing maximum phosphorylation. (C) Cells were incubated with 25 μM of the PI3K inhibitor LY294002 for 1 hr, followed by treatment with 10−7 M insulin for the indicated time-points, and Akt phosphorylation was detected by Western blot analysis, as mentioned above. Insulin-induced Akt phosphorylation was abrogated by pre-treatment with a PI3K inhibitor. (D) Cells were incubated with 25 μM of the PI3K inhibitor LY294002 for 1 hr, followed by treatment with 10−7 M insulin. The migration distances were measured at 24 and 48 hrs after treatment. Insulin-induced endothelial cell migration was abrogated by pre-treatment with a PI3K inhibitor. The results are representative of at least three independent experiments. **P < 0.01 versus control; ***P < 0.001 versus control. (E) Cells were either left untreated as a control or treated with 25 μM of LY294002 for 1 hr, then treated with or without 10−7 M insulin, and the tube formation assay was performed. Each treatment group was performed in triplicate. Insulin-induced tube formation was prevented by pre-treatment with the PI3K inhibitor.
