Figure 7.
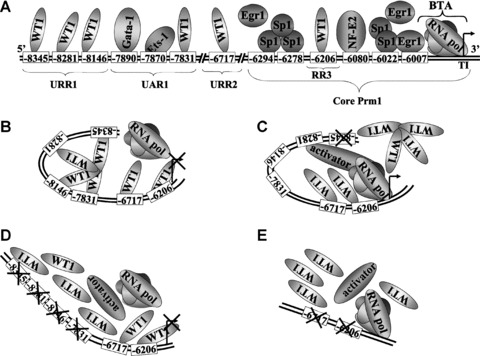
Proposed model for WT1-mediated repression of Prm1 in HEL 92.1.7 cells. (A): Schematic representation of the relative positions of functional binding elements within Prm1 (not drawn to scale), as well as binding of the basal transcription apparatus (BTA) to the transcription initiation site. Overlapping Sp1/Egr1 elements at –6294, –6278, –6022 and –6007, as well as an NF-E2 element at –6080, located within the ‘core’ proximal promoter, direct efficient basal activity of Prm1 in megakaryoblastic HEL cells. Additionally, GATA-1 and Ets-1 bind elements at –7890 and –7870, respectively, within UAR1 to increase Prm1 activity in HEL cells [41]. The data herein indicate that WT1 binds to GC elements within URR1, specifically at –8345, –8281 and –8146, as well as elements at –7831 within UAR1, –6717 within URR2 and –6206 within RR3, to repress Prm1 activity. (B, C, D and E): Proposed model for WT1- mediated repression of Prm1 in HEL cells. It is suggested that WT1 overcomes competition from other factors, such as Egr1 and Sp1, by binding cooperatively to neighbouring GC elements at –8345, –8281, –8146 and –7831 and independently to GC elements at –6717 and –6206 to mediate repression of Prm1-directed transcription by the basal transcription apparatus in HEL cells (B). Mutation of any of the upstream GC elements at –8345, –8281, –8146 and –7831 by site-directed mutagenesis interferes with cooperation among WT1 proteins binding to these elements, thereby inhibiting WT1 binding and alleviating repression of Prm1. In the absence of repressor binding to the remaining intact sites, these elements may now have a higher affinity for activating factors (C). Disruption of remaining upstream GC elements blocks the binding of activators and results in de-activation of the promoter (D). Furthermore, mutation of GC elements at –6717 and –6206 in Prm1D (–6848) alleviates repression of Prm1 (E).
